��Ŀ����
��ҵ�����ý�̿��ʯ��Ҥ��ȼ�շ��ȣ�ʹʯ��ʯ�ֽ�����CO2����Ҫ��Ӧ���£�
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������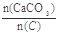 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
��3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ�������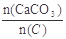 Ϊ��ֵ��
Ϊ��ֵ��
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������
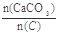 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ����3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ�������
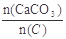 Ϊ��ֵ��
Ϊ��ֵ����1��425.6 ��2��44.4%��3��1.90
�����������1�����ݷ�Ӧ�ڵĻ�ѧ����ʽ��֪CO2�����ʵ�������CaCO3�����ʵ��������Ա�״����CO2�����Ϊ��2.0��95%��106g��100g/mol��22.4L/mol=425600L����425.6m3��
��2����n��C��=1mol����n��CaCO3��=2.2mol���������е�O2��C��ȫ��Ӧʱ������Ҥ����CO2����������1molCȼ������1mol CO2��2.2mol CaCO3�ֽ�����2.2mol CO2������CO2���������=��1+2.2���£�1+2.2+4����100%=44.4%
��3����Ҥ��Ϊ100mol����n��N2��=100mol-41.6mol-0.2mol-0.2mol=58mol�����Է�ӦǰO2������Ϊ��58mol��4=14.5mol���μӷ�Ӧ��O2Ϊ��14.5mol-0.2mol=14.3mol��2C+O2=2CO������0.2mol CO��O2 0.1mol��C+O2=CO2����C��Ӧ����CO2��O2Ϊ��14.3mol-0.1mol=14.2mol�����ɵ�CO2Ϊ14.2mol��CaCO3�ֽ����ɵ�CO2Ϊ��41.6mol-14.2mol=27.4mol����CaCO3�����ʵ���Ϊ27.4mol������Ҥ�������
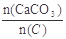 ��27.4mol����0.2mol+14.2mol��=1.90
��27.4mol����0.2mol+14.2mol��=1.90
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �к��е���ԭ������2��6.02��1023
�к��е���ԭ������2��6.02��1023 ��6��02��1023��
��6��02��1023��