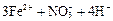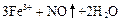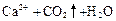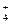��Ŀ����
��15�֣����п�ͼ�е�A ~ K���ʾ�Ϊ��ѧ�������ʡ���֪B��D��E��L������Ϊ�ܶȱȿ���������壬D��EΪ���ʣ�����Ϊ�����A��I���dz��õ�Ư����F����ɫ��Ӧ�ʻ�ɫ��F��G������L��ˮ��Һ��Ӧ�ų�B�������ͼʾ��ϵ�ش����⣺
��1��F�������ǣߣߣߣߣߣߣ�K�ĵ���ʽ�ߣߣߣߣߣߡ�
��2����Ӧ��~���У�������������ԭ��Ӧ���ǣߣߣߣߣߣߡ�
��3����Ӧ�ܵ����ӷ���ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�۵Ļ�ѧ����ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ij��������H��Ӧ����E���÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߡ�

��1��F�������ǣߣߣߣߣߣߣ�K�ĵ���ʽ�ߣߣߣߣߣߡ�
��2����Ӧ��~���У�������������ԭ��Ӧ���ǣߣߣߣߣߣߡ�
��3����Ӧ�ܵ����ӷ���ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�۵Ļ�ѧ����ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ij��������H��Ӧ����E���÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߡ�
����15�֣�w.&@m
��1�����2�֣�����ʽ�ԣ�2�֣�
��2���٢ۢݣ�2�֣���©ѡһ���1�֣�©ѡ�����ѡ�����÷֣�
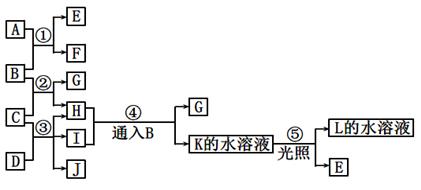
��3��Ca2����2ClO-��CO2��H2O ="=" 2HClO��CaCO3����3�֣�
2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O ��3�֣�
��4��2F2��2H2O==O2��4HF ��3�֣�
��1�����2�֣�����ʽ�ԣ�2�֣�
��2���٢ۢݣ�2�֣���©ѡһ���1�֣�©ѡ�����ѡ�����÷֣�
��3��Ca2����2ClO-��CO2��H2O ="=" 2HClO��CaCO3����3�֣�
2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O ��3�֣�
��4��2F2��2H2O==O2��4HF ��3�֣�
��

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
 ��
��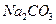 ��Һ�м���0.01 mol
��Һ�м���0.01 mol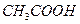 ��
��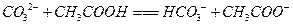
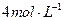 ��
��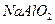 ��Һ��
��Һ��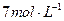 �������������Ȼ�ϣ�
�������������Ȼ�ϣ�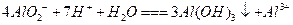
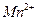 ����Һ�м���
����Һ�м���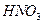 �ټ���
�ټ���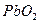 ����Ӧ��ϵ���Ϻ�ɫ��
����Ӧ��ϵ���Ϻ�ɫ��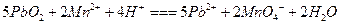
 Al(OH)3 (����) + 3H+
Al(OH)3 (����) + 3H+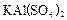 ��Һ�м���
��Һ�м���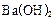 ��ʹ
��ʹ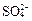 ǡ����ȫ������
ǡ����ȫ������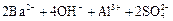

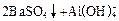
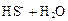


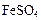 ��ϡ���ᷴӦ��
��ϡ���ᷴӦ��