��Ŀ����
����Ŀ����(Se)�ǵ�34��Ԫ�أ��������ڲ��ɻ�ȱ����Ԫ�أ������γ�H2Se��SeO2��H2SeO3��H2SeO4��CuSe�ȶ��ֻ������ش��������⣺

��1������Ԫ�����ڱ��е�λ��________________________��
��2����101kPa��һ���¶�(һ����298K)�£����ȶ����ʷ�����Ӧ����1mol������ķ�Ӧ�Ƚиû�����ı�������(��fH��)��ͼ1Ϊ����Ԫ���⻯��a��b��c��d����̬ʱ�����������ݡ�
��ͼ1���⻯��d�ĵ���ʽΪ__________________________��
����298Kʱ��������ֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ__________________________��
����ͼ�����ݼ��㣬2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g) ��H=_____________KJ/mol
2Se(s)+2H2O(g) ��H=_____________KJ/mol
��3���ں��ݷ�Ӧ���У���H2Se(g)��O2(g)����ͬ����[n(H2Se)/n(O2)=m]Ͷ�뷴Ӧ������÷�Ӧ2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g)��H2Se��ƽ��ת�������¶ȱ仯��ͼ2��ʾ����A��B����ƽ�ⳣ����С��ϵΪKA________KB(����<������>������=��)��ͼ��m1��m2��m3�ɴ�С��˳��Ϊ ____________��������____________________________________��
2Se(s)+2H2O(g)��H2Se��ƽ��ת�������¶ȱ仯��ͼ2��ʾ����A��B����ƽ�ⳣ����С��ϵΪKA________KB(����<������>������=��)��ͼ��m1��m2��m3�ɴ�С��˳��Ϊ ____________��������____________________________________��
��4���������ܶȻ���Ksp(CuSe)=7.9x10-49��Ksp(CuS)=1.3��10-36����ӦCuS(s)+Se2-(aq) ![]() CuSe(s)+S2-(aq)�Ļ�ѧƽ�ⳣ��KΪ____________(����ÿ�ѧ��������ʾ��������2λС��)������Һ��c(S2-)=100c(Se2-)ʱ����Ӧ��v(��)_____v����)(����<������>������=��) ��
CuSe(s)+S2-(aq)�Ļ�ѧƽ�ⳣ��KΪ____________(����ÿ�ѧ��������ʾ��������2λС��)������Һ��c(S2-)=100c(Se2-)ʱ����Ӧ��v(��)_____v����)(����<������>������=��) ��
���𰸡� �������ڢ�A�� ![]() H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol -646 > m3>m2>m1 ��ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת�������� 1.65��1012 >
H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol -646 > m3>m2>m1 ��ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת�������� 1.65��1012 >
����������1������Ԫ�����ڱ��е�λ��Ϊ������������A�壻
��2���ǽ���Ԫ���⻯����ȶ���������1mol�⻯��ʱ����H�Ĺ�ϵΪ������Ԫ�������ɣ�ͬһ����Ԫ�طǽ�����Խǿ��������̬�⻯��Խ���ף���̬�⻯��Խ�ȶ�������������ѧ������Խ��Խ�ȶ���a��b��c��d����ΪH2Te��H2Se��H2S��H2O��
��d�ĵ���ʽΪ![]() ��
��
����ͼ1��֪����298Kʱ�����������������81kJ/mol�����Էֽ�����H=-81kJ/mol���ʷֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ��H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol
����ͼ1��֪��H2O�������ȵ��ȷ���ʽΪH2+1/2O2=H2O ��H=-242kJ/mol��1����H2Se�ֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ��H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol��2�������ݸ�˹���ɣ���1����2+��2����2�ɵ�Ŀ�귽��ʽ������H=-242��2-81��2=-646 kJ/mol��
��3����ͼ2��֪������ת���ʽ��ͣ�������ƽ�ⳣ����С��B���¶ȸ���A�㣬��KA>KB����ͬ�¶��£�H2Se��ƽ��ת����m3<m2<m1�����n(H2Se)/n(O2)=m����ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת������������m3>m2>m1��
��4��������֪����ʽ���Եó���ѧƽ�ⳣ�� ������ѧƽ��ʱ��
������ѧƽ��ʱ��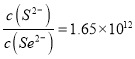 ������ʱ��Һ��
������ʱ��Һ�� ��Ӧ��û��ƽ�Ⲣ��v(��)>v����)���ʴ�Ϊ��1.65��1012�� >��
��Ӧ��û��ƽ�Ⲣ��v(��)>v����)���ʴ�Ϊ��1.65��1012�� >��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����Ŀ������ʵ������У��ɴﵽ��Ӧʵ��Ŀ�ĵ���( )
ʵ����� | ʵ��Ŀ�� | |
A | ������ˮ��Ϻ�������� | ���屽 |
B | ij�л�����������Ȼ�̼��Һ��� | ȷ�ϸ��л��ﺬ̼̼˫�� |
C | �� | ������л����е���ԭ�� |
D | �Ҵ������Ը��������Һ��� | �����Ҵ����л�ԭ�� |
A. A B. B C. C D. D