��Ŀ����
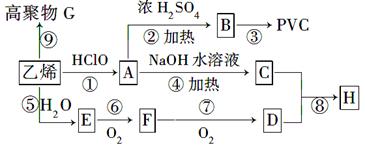
��ҵ������ϩ������Ϊԭ�Ϻϳɾ�����ϩ(PVC)����֪�������ܸ���ϩ�����ӳɷ�Ӧ��CH2��CH2��HClO��CH2(OH)CH2Cl������ϩΪԭ����ȡPVC�Ȳ�Ʒ��ת����ϵ��ͼ��ʾ��
�Իش��������⣺
��1��д���л���B��G�Ľṹ��ʽ��B____________��G_________��
��2���ڡ��ݡ��ߵķ�Ӧ���ͷֱ���_______________��_____________��________________��
��3��д��D��һ��ͬ���칹��Ľṹ��ʽ_____________��
��4��д����Ӧ�Ļ�ѧ����ʽ__________________________________��
��5��E�ĺ˴Ź��������������壬�����֮��Ϊ��______________����С����
��6��д��C��D�����ʵ���֮��Ϊ1��2��Ӧ����H�Ļ�ѧ����ʽ��
______________________________________________________________��
��15�֣���1��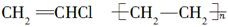 ������2�֣�
������2�֣�
��2����ȥ��Ӧ���ӳɷ�Ӧ����������Ӧ������1�֣�
��3��HCOOCH3������CH2(OH)CHO����2�֣�
��4��2CH3CH2OH��O2Cu��2CH3CHO��2H2O��2�֣�����5��1����2����3����2�֣�
��6��CH2(OH)CH2(OH)��2CH3COOH 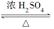 CH3COOCH2CH2OOCCH3��2H2O��2�֣�
CH3COOCH2CH2OOCCH3��2H2O��2�֣�
��������
�����������1��������֪ѡ���֪��A�Ľṹ��ʽ��CH2��OH��CH2Cl ������A�й����ŵ��������ǻ�����ԭ�ӡ�A������ȥ��Ӧ����B����B�Ľṹ��ʽ��CH2=CHCl.��ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ���ɾ���ϩ���ṹ��ʽ�� ��
��
��2����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����E���Ҵ�����F����ȩ����ȩ����������Ӧ�������ᣬ��D�����ᣬ���������ͬ���칹��Ľṹ��ʽ��HCOOCH3��CH2(OH)CHO��
��3��D�����ᣬ���������ͬ���칹��Ľṹ��ʽ��HCOOCH3��CH2(OH)CHO��
��4����Ӧ�����Ҵ��Ĵ����������Է�Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH��O2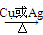
2CH3CHO��2H2O��
��5�������Ҵ��Ľṹ��ʽCH3CH2OH��֪���˴Ź��������������壬�����֮��Ϊ1:2:3��
��6�����ݷ�Ӧ�ܵ�������֪���÷�Ӧ��±������ˮ�ⷴӦ������C���Ҷ�����������ᷢ��������Ӧ�ķ���ʽ��CH2(OH)CH2(OH)��2CH3COOH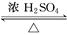 CH3COOCH2CH2OOCCH3��2H2O��
CH3COOCH2CH2OOCCH3��2H2O��
���㣺�����л���ṹ��ʽ�������ŵ��ж��Լ���ѧ����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ�����ȷ���ֹ����Žṹ�����ʣ�Ȼ��������������⡢����������ɣ�����������ѧ���Ĺ淶����������Ӧ��������



 ��CO
��CO
 CH2Cl-CH2Cl
CH2Cl-CH2Cl CH2=CHCl+HCl
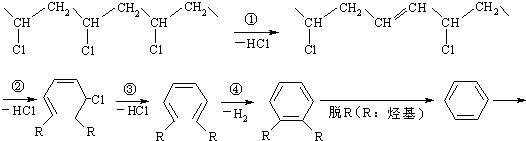
CH2=CHCl+HCl �ڱ�������������DOP���ǹ��ұ�������ʹ�õ����ܼ�֮һ���ڱ���������������DOP��ԭ�ϣ����������ļ״���Ӧ�ܵõ���һ�����ܼ�DMP
�ڱ�������������DOP���ǹ��ұ�������ʹ�õ����ܼ�֮һ���ڱ���������������DOP��ԭ�ϣ����������ļ״���Ӧ�ܵõ���һ�����ܼ�DMP


 +3HNO3
+3HNO3 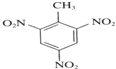 +3H2O
+3H2O