��Ŀ����
[��ѧ����ѡ��5���л��ﻯѧ����]��15�֣�
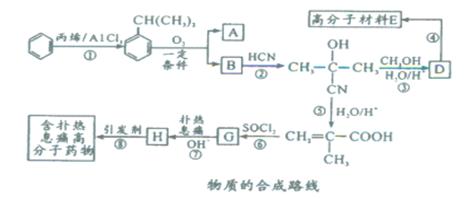
�߷��Ӳ���E�ͺ�����Ϣʹ�߷���ҩ��ĺϳ�������ͼ��ʾ��
��֪��I��������Ϣʹ�߷���ҩ��ĽṹΪ
�Իش��������⣺
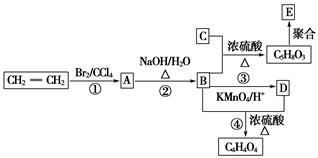
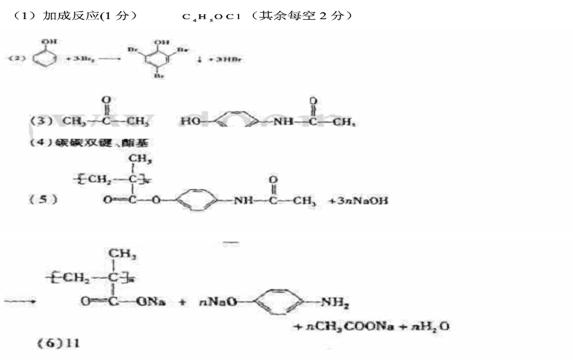
��1����Ӧ�ٵķ�Ӧ����Ϊ ��G�ķ���ʽΪ ��
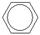
��2����1mol CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ ������Ϣʹ�Ľṹ��ʽΪ ��
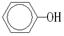
��4��D�����ܶ�����ͬ״̬�¼����ܶȵ�6��25����D�и�Ԫ�ص����������ֱ�Ϊ̼60%����8%����32%��D����������������Ϊ ��
��5��д��������Ϣʹ�߷���ҩ��������������Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��6��D�ж���ͬ���칹�壬������D������ͬ���������ܷ���������Ӧ��ͬ���칹����
�֣�����˳���칹����
�߷��Ӳ���E�ͺ�����Ϣʹ�߷���ҩ��ĺϳ�������ͼ��ʾ��

��֪��I��������Ϣʹ�߷���ҩ��ĽṹΪ

�Իش��������⣺
��1����Ӧ�ٵķ�Ӧ����Ϊ ��G�ķ���ʽΪ ��
��2����1mol
 CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ����3����Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ ������Ϣʹ�Ľṹ��ʽΪ ��
��4��D�����ܶ�����ͬ״̬�¼����ܶȵ�6��25����D�и�Ԫ�ص����������ֱ�Ϊ̼60%����8%����32%��D����������������Ϊ ��
��5��д��������Ϣʹ�߷���ҩ��������������Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��6��D�ж���ͬ���칹�壬������D������ͬ���������ܷ���������Ӧ��ͬ���칹����
�֣�����˳���칹����
����������ŷ�Ӧ��Ϊ
 +CH2=CH-CH3 ��
+CH2=CH-CH3 ��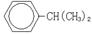 �����ڼӳɷ�Ӧ��������֪���֪G�Ľṹ��ʽΪCH2=C(CH3)-COCl�������ʽΪC4H5OCl��
�����ڼӳɷ�Ӧ��������֪���֪G�Ľṹ��ʽΪCH2=C(CH3)-COCl�������ʽΪC4H5OCl�� �ƾ����⡰
 ��
�� ��A��+
��A��+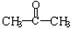 ��B��������A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽΪ��
��B��������A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽΪ��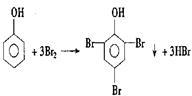 ��
�������ڷ�Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ
 �����ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ ��
�����ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ ��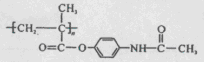 ����ȷ��H�Ľṹ��ʽΪ
����ȷ��H�Ľṹ��ʽΪ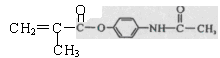 ���ٽ��G�Ľṹ��ʽ[CH2=C(CH3)-COCl]��������Ϣʹ�Ľṹ��ʽΪ
���ٽ��G�Ľṹ��ʽ[CH2=C(CH3)-COCl]��������Ϣʹ�Ľṹ��ʽΪ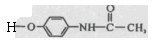 ��
����D��Ħ������M��6.25��16��100g/mol���ɴ˿�ȷ���������������ԭ�����ֱ�ΪN��C����
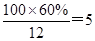 ��N��H����
��N��H���� ��N��O����
��N��O����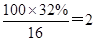 ������ʽΪC5H8O2�����ӵIJ����Ͷ�Ϊ2���������ͼ����Ϣ��ȷ��D�Ľṹ��ʽΪCH2=C(CH3)-COOCH3��������������Ϊ̼̼˫����������
������ʽΪC5H8O2�����ӵIJ����Ͷ�Ϊ2���������ͼ����Ϣ��ȷ��D�Ľṹ��ʽΪCH2=C(CH3)-COOCH3��������������Ϊ̼̼˫�����������ɸ��ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ��
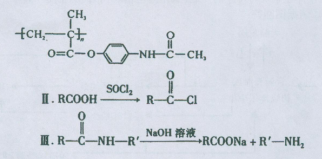
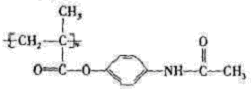 ������ÿ�������к���������������Һ��Ӧ�Ĺ�������1���������ɷ��ǻ��γɵ���������1���ļ����ݴ˱��д���÷�Ӧ����ʽ��
������ÿ�������к���������������Һ��Ӧ�Ĺ�������1���������ɷ��ǻ��γɵ���������1���ļ����ݴ˱��д���÷�Ӧ����ʽ���ʸ���D�ķ���ʽΪC5H8O2���䲻���Ͷ�Ϊ2���ٸ�����������ȷ����ͬ���칹����Ҫ����̼̼˫������OOCH����ͬ���칹��ֱ��У�CH2��CH��CH2��CH2��OOCH��CH3��CH��CH��CH2��OOCH����˳���ṹ����CH3��CH2��CH��CH��OOCH����˳���ṹ����CH2��C(CH3)��CH2��OOCH��(CH3)2C��CH��OOCH��CH2��CH��CH(CH3)��OOCH��CH3��CH��C(CH3)��OOCH����˳���ṹ����CH2��C(C2H5)��OOCH����11�֡�

��ϰ��ϵ�д�
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ
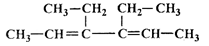 ��A�ĺϳ�·������ͼ������B��H�ֱ����һ���л��
��A�ĺϳ�·������ͼ������B��H�ֱ����һ���л��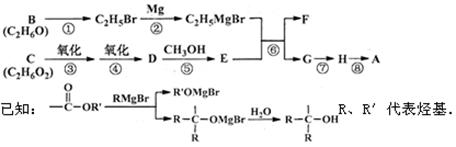

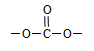 �ṹ ��
�ṹ ��