��Ŀ����
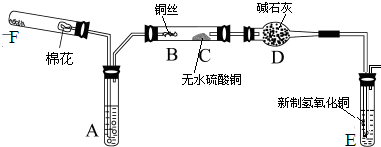
��10�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ������������������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��
ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1)������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ(��������ij��λ�Ĵ���) ��
(2)ΪʹA���Ҵ�ƽ���������Ҵ�����,�����õķ�����_____________��
D��ʹ�ü�ʯ�ҵ������� ��
(3)�����Ҵ���������ʱF�е�ʵ�������� ��
(4)E����һ�ִ�����䷴Ӧ����ʽΪ ��
(5)д���Ҵ������������Ļ�ѧ����ʽ ��
.��10��
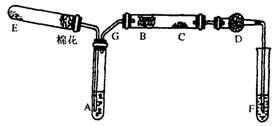
��1��E��A��B��F��©ѡ��1�֣���ѡ����ѡ���÷֣� ����2�֣�
��2��ˮԡ���� ��1�֣�
��ֹF�е�ˮ��������C������ˮCuSO4���ã�Ӱ�����ˮ�ļ��顣��2�֣�
��3��F�в�����ɫ��������1�֣�
��4��2KMnO4=K2MnO4+MnO2+O2������2�֣�
��5��2CH3CH2OH+O2��2CH3CHO+2H2O��2�֣�
����:

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�