��Ŀ����
�����ȷ�Ӧ���Ļ�ѧ����ʽΪ��2Al+Fe2O3 Al2O3+2Fe��ijͬѧ�ԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۹�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
Al2O3+2Fe��ijͬѧ�ԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۹�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���______�����۲쵽______����ʱ��˵���������к��н���������Ӧ�����ӷ���ʽΪ______��
��3��ʵ�����ܽ����������ѡ�������Լ��е�______ ������ţ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ��
��2���������ܺ��������Ʒ�Ӧ�ų����������������������Ʋ���Ӧ��
��3�����ܺͽ�������Ӧ���ܺͽ�������Ӧ��������ϡ���ᣬ���һ�õIJ�������Ⱦ��
����⣺��1�����ݱ��е����ݿ��Կ������������ķе�����ͣ�����������Һ̬ʱ��������Ҳ��Һ̬���������ȷ�Ӧ�õ����ǽ������ͽ������Ļ����ʴ�Ϊ���������ķе�����ͣ�
��2���������ܺ��������Ʒ�Ӧ�ų����������������������Ʋ���Ӧ����Ӧʵ���ǣ�2Al+2OH-+2H2O=2AlO2-+3H2��������������������Һ֤���������õĿ�״�������к��н�������
�ʴ�Ϊ������������Һ�������ݲ�����2Al+2OH-+2H2O=2AlO2-+3H2����
��3�����ܺͽ�������Ӧ���ܺͽ�������Ӧ��������ϡ���ᣬ���һ�õIJ�������Ⱦ��Ũ�����ڳ����¿��Ժͽ������ۻ���ϡ������Ժͽ�����Ӧ�����ж���һ���������壬��������ֻ�ͽ�������Ӧ���ͽ���������Ӧ����ѡB��
���������⿼��ѧ���������Ļ�ѧ���ʣ����Ը�����ѧ֪ʶ���лش��ѶȲ���

��12�֣�ijͬѧ�ԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��. ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺

��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ������Ϊ�� ��
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ�Ϊ ��
��3����c=13mLʱ��ԭ��Һ��Fe3+��Al3+�����ʵ���֮��Ϊ ��
��12�֣�ijͬѧ�ԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
|
���� |
Al |
Al2O3 |
Fe |
Fe2O3 |
|
�۵�/�� |
660 |
2054 |
1535 |
1462 |
|
�е�/�� |
2467 |
2980 |
2750 |
-- |
I.��ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ��������һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ��� ����Ӧ�����ӷ���ʽΪ ��
��. ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
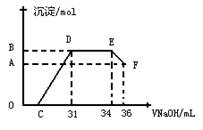
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ������Ϊ�� ��
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ�Ϊ ��
��3����c=13mLʱ��ԭ��Һ��Fe3+��Al3+�����ʵ���֮��Ϊ ��
 ��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ
��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ