��Ŀ����
��16�֣����������(Na2S2O3)������Ҫ����������Σ��׳ơ������������������մ�������ˮ���������Ҵ�������֯��Ư�ס������������й㷺Ӧ�á�
ijУ��ѧ�о���ѧϰС���������ѧϰ��˼�룬ͨ��ʵ��̽��Na2S2O3�Ļ�ѧ���ʡ�
����Ʒ�Ʊ���ʵ�����г����������ƺ�����Ʊ�Na2S2O3��5H2O��д����Ӧ�Ļ�ѧ����ʽ ��
��ӦҺ����ɫ�����ˡ�Ũ���ᾧ�����ˡ�ϴ�ӡ����T�ò�Ʒ�����þ������Ҵ�ϴ�ӵ�Ŀ���� ��
��������⡿Na2S2O3�Ƿ���Na2SO4�߱����Ƶ����������أ�
����٣� ��
����ڣ���Һ�����ԣ��Ҳ����ᷴӦ��
����ۣ���ԭ�ԣ����ܱ�������������
������̽����������������ڡ��ۣ����ʵ�鷽����
|
|
ʵ����� |
ʵ������� Ԥ��ʵ������ |
������ͣ��� ���ӷ���ʽ��ʾ�� |
|
����� |
������ֽ�����ɫ������
|
��ҺpH=8 |
|
|
��pH=2�������� �μ�Na2S2O3��Һ |
|
2S2O32- +2H+ �T�T S��+ SO2 ��+ H2O |
|
|
����� |
��������ˮ�еμ�����Na2S2O3��Һ |
��ˮ��ɫ��dz |
|
��ʵ����ۡ�
Na2S2O3�����ᷴӦ�����л�ԭ�ԣ���Na2SO4�Ļ�ѧ���ʲ����ơ�
���������ۡ�
��1����ͬѧ��̽��������ۡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷����Ƿ���ȷ��˵�����ɣ� ��
��2�����������һ��ʵ�鷽����֤��Na2S2O3���л�ԭ�ԡ����ʵ�鷽���ǣ� ��
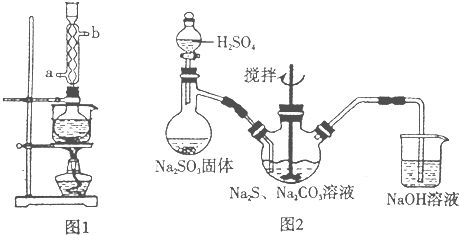
����Ʒ��ȡ��Na2SO3 + S + 5H2O �T�TNa2S2O3��5H2O ��ȥ���������ʣ����ٲ�Ʒ���ܽ����
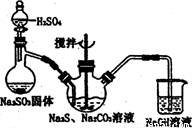
��������⡿��BaCl2��Һ��Ӧ�г�������
������̽�����ò�����պȡNa2S2O3��Һ����ε�pH��ֽ�����룬�е���ɫ��������ɫ�̼�����ζ������� S2O32-��4Cl2��5H2O=2SO42-��8Cl����10H��
���������ۡ�
��1������ȷ������ˮ�б����ͺ���Cl��
��2��������Na2S2O3��Һ�е�����ˮ��Ȼ������Ȼ�����Һ�����۲쵽��ɫ��������������������ܽ⣬��˵��Na2S2O3�ܱ���ˮ����
��������
�������������Ʒ��ȡ������ȷ��Ӧ����Na2SO3 ��S ����������Na2S2O3��5H2O���ɿ�����д����ѧ��Ӧ����ʽ������֪����������ˮ���������Ҵ�����˵���Ҵ��������dz�ȥ���ʡ�������ġ�
��������⡿���������Ȼ�����Ӧ���ɰ�ɫ����������Na2S2O3��BaCl2��Һ��Ӧ�г������ɣ�
������̽������������ˮ��Ĺ��ɣ�������ˮ�⣬������ˮ�⣬Խ��Խˮ�⣬˭ǿ��˭�ԣ�Na2S2O3Ϊ
ǿ�������Σ�ˮ��ʼ��ԣ���PH��ֽ����Һ��PH��
��pH=2�������еμ�Na2S2O3��Һ����������ƺ����ᷴӦ���ɶ���������������ʣ�Na2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O��
����������ԭ��Ӧ��ʵ�ʵ�ʧ�����غ㣬S2O32-��2SO42-��8e-��Cl2��2CI-��2e-������4molCl2����1molS2O32-���õ�8molCl-��2molSO42-�����ݵ���غ㣬��������Ӧ��10molH+������ԭ���غ㷴Ӧ����Ӧ��5molH2O�����ӷ���ʽΪS2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
���������ۡ���1����ˮ�ijɷ��к��������ӣ����Ժ�AgNO3��Һ��Ӧ������ɫ������
��2��Na2S2O3����ˮ����������������ӣ����������ʱҪ�ų�̼������Ӻ�����������ӵĸ��ţ����Լ����������ữ���Ȼ�����Һ������а�ɫ������������֤��������������ӣ���֮��û�С�
���������ʵļ��飬Ҫ���������и�����Ϣ�������⡢������⣬��Ŀ�Ѷ��еȣ�
���㣺���������龳�»�ѧ����ʽ����д��ʵ�鷽������ơ����롢��֤�����������Ϣ���������ʵ���������������Ŀ�Ѷ��еȡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�