��Ŀ����
ѡ����(��������ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ�γ�ģ������ݣ�����ѡ������һ�������������ȫ��������һ�����֡�(A)HN3��Ϊ�����ᣬ������Ϊ��ɫ�д̼�����ζ��Һ�塣![]() Ҳ����Ϊ��±���ӡ�������������Ʒ�Ӧ���Ƶõ����ᡣ���������ƿɴ����з�Ӧ�Ƶã�
Ҳ����Ϊ��±���ӡ�������������Ʒ�Ӧ���Ƶõ����ᡣ���������ƿɴ����з�Ӧ�Ƶã�
NaNH2+N2O![]() NaN3+H2O��HN3��Ũ������Һ���ܽ�ͭ��������Ȳ����ý��������ܽ�ͭ����
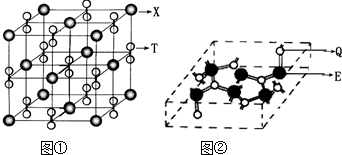
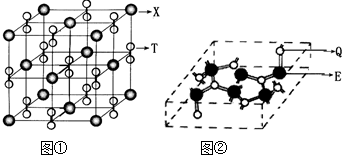
NaN3+H2O��HN3��Ũ������Һ���ܽ�ͭ��������Ȳ����ý��������ܽ�ͭ����![]() ��ͭ�Ͳ��Ļ������ڳ�����ҽҩ������ҪӦ�ã�Cu�Ļ�����A(������ͼ)��Ϊ����������֮һ������ѧʽΪPt(NH3)2Cl2�Ļ������������칹�壬����B�칹����п����ԣ����������ư�֢���Իش��������⣺
��ͭ�Ͳ��Ļ������ڳ�����ҽҩ������ҪӦ�ã�Cu�Ļ�����A(������ͼ)��Ϊ����������֮һ������ѧʽΪPt(NH3)2Cl2�Ļ������������칹�壬����B�칹����п����ԣ����������ư�֢���Իش��������⣺

(1)��̬��ԭ�Ӻ�������Ų��Ĺ����ʾʽΪ_____________��
(2)Ԫ��N��S��P�ĵ�һ������(I1)�ɴ�С��˳��Ϊ_____________��
(3)HN3����_____________���壬![]() �Ŀռ乹����_____________����
�Ŀռ乹����_____________����![]() ��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ_____________(д1��)��
��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ_____________(д1��)��![]() �ĵ���ʽΪ____________��������ԭ�ӵ��ӻ�������____________��
�ĵ���ʽΪ____________��������ԭ�ӵ��ӻ�������____________��
(4)![]() �еļ���Ϊ___________������������A�Ļ�ѧʽΪ___________��
�еļ���Ϊ___________������������A�Ļ�ѧʽΪ___________��
(5)�ΰ�ҩ��B�Ľṹ��ʽΪ___________��
(B)���������̷���̼����狀��Ȼ���Ϊԭ���Ʊ������г��Ͻ��ε�����ز�Ʒ���¹��ա���֪����Ӧ(��)�Ļ�ѧ����ʽΪ��FeSO4��7H2O+2NH4HCO3![]() FeCO3��+(NH4)2SO4+CO2��+H2O
FeCO3��+(NH4)2SO4+CO2��+H2O
��Ӧ(��)�Ļ�ѧ����ʽΪ��(NH4)2SO4+2KCl![]() K2SO4+2NH4Cl
K2SO4+2NH4Cl
�����������£�

�Իش��������⣺
(1)�ù����еĸ���Ʒ��____________��(�ѧʽ)
(2)����C������Ϊ____________��
(3)ԭ���̷���̼����淋����Ͷ�ϱ���____________(������)ʱ��FeSO4��ת���ʴ���95%��
(4)���ʼ���____________(�ѧʽ)����Ӧ(��)�Ǽ������������������գ��¶�Ϊ700��
(A)
(1)
(2)N��P��S
(3)���� ֱ�� CO2 (N2O��д1������) ![]() sp3
sp3
(4)��λ��(д���ۼ�Ҳ��) YBa2Cu3O7
(5)
������(1)��̬��ԭ�ӵĹ����ʾʽΪ�� ��
��
(3)�ȵ�����Ҫ������������ԭ����Ҳ��ȡ�
����![]() �ĵȵ�������CO2��N2O�ȡ�
�ĵȵ�������CO2��N2O�ȡ�
(4)����������A��
n(Y)��n(Ba)��n(Cu)��n(O)=![]() ��8��8��
��8��8��![]() ��3��(10��
��3��(10��![]() +2)=1��2��3��7
+2)=1��2��3��7
��A�Ļ�ѧʽΪYBa2Cu3O7��
(5)������BӦ�Ǽ��Է��ӣ����Խṹ��ʽΪ ��
��
(B)(1)Fe2O3��NH4Cl
(2)����
(3)278��79
(4)FeCO3 4FeCO3+O2![]() 2Fe2O3+4CO2
2Fe2O3+4CO2