��Ŀ����
��12�֣�Ϊ����֤Cu�� ŨH2SO4��Ӧ�IJ�������SO2��H2O��ѡ����ͼ��ʾ����(�ں�����)��װ��ʵ��װ��: B������ˮ����ͭ��C����Ʒ����Һ��D��������������Һ
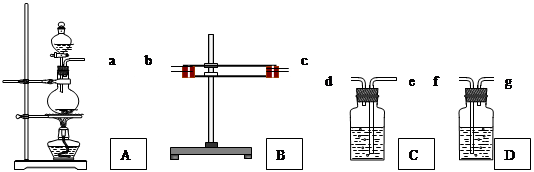
(1) �簴������������������������װ�õ���ȷ˳����(����ӿ���ĸ)��
a�� �� �� �� �� ��
(2) ����B��CӦ��������ʵ������ű����Ѽ����SO2��H2O?
B�� ��C�� ��
���� B��Cװ��ǰ��Ե�����ʵ���к�Ӱ��
��
(3) D������������Һ�������� ��
(4) д��A�з�Ӧ�Ļ�ѧ����ʽ .
����12�֣�
(1) a�� b����c�� �� c����b�� �� e �� d �� f ������1�֣���5�֣�
(2) B�� ��ɫ��ĩ���� ��C�� Ʒ����ɫ ������1�֣���2�֣�
���� B��Cװ��ǰ��Ե�����ʵ���к�Ӱ�� ���Cװ�÷���B֮ǰ�����ܼ������Ӧ�Ƿ���ˮ���ɡ� ��2�֣�
(3)D������������Һ�������� ���ն����SO2����ֹ��Ⱦ���� ����1�֣�
(4)д��A�з�Ӧ�Ļ�ѧ����ʽ 2H2SO4��Ũ��+Cu === CuSO4 +SO2��+2H2O . ��2�֣�
����:

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
