��Ŀ����
��14�֣��������ԭ�����ش����и�С�⣺
��֪��![]() ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ![]()
(1)�����£�PH=5��![]() ��Һ��ˮ�ĵ���̶� PH=9��
��Һ��ˮ�ĵ���̶� PH=9��![]() ��ˮ�ĵ���̶ȡ����>������=����<����
��ˮ�ĵ���̶ȡ����>������=����<����
��2������������ʵ���Ũ�ȵ�![]() �백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������
�백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������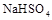 ��Һ�백ˮ��Ϻ���ҺPH=7����
��Һ�백ˮ��Ϻ���ҺPH=7����
![]()
![]() ���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��
���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��![]() ��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
(3)���ֱ���![]() ��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
����![]() ������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
�������û��Һ����ɫ������������������Һ��һ�����ڵ��� ��
һ�������ڵ������� ��
��14�֣�
(1)= ��2�֣�
(2)![]() (2��) =��2�֣� PH>7��1�֣�
(2��) =��2�֣� PH>7��1�֣�
(3)��![]() ��1�֣���
��1�֣���![]() ��2�֣���
��2�֣���![]() ��2�֣�
��2�֣�
����:

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
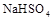 ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ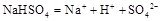
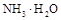 ��ˮ�ĵ���̶ȡ����>������=����<����
��ˮ�ĵ���̶ȡ����>������=����<����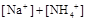
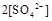 ���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��
���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��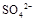 ��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
��ȫ��������Ӧ����Һ��PH= 7���>������=����<����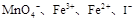 ��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ�� ������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���