��Ŀ����
Ŀǰ�ⶨ������SO2������Ҫ��������ԭ��Ӧ����֪SO2����������KMnO4��Һ��Ӧʱ��MnO4- ����ԭΪMn2+��SO2��������SO42-��
Ϊ�ⶨij�ط��Ŀ�����SO2�Ϳ���������ĺ�������ͬѧ�������ͼ��ʾ��ʵ��װ�ã�

������ ��
�� ��ʾ�ܱ�������
��ʾ�ܱ�������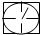 ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ���������
������KMnO4��Һ���Ϊ200mL��Ũ��Ϊ0.1mol?L-1���ش��������⣺
��1������200mL0.1mol?L-1����KMnO4��Һ�����õ���������ʹ�õ��Ⱥ�˳��������______����������______��______��______����������������______��
��2��д������ƽ�ⶨSO2���������ӷ���ʽ��______
��3�����������ٹ�����������Ϊacm3/min����tminʱ����KMnO4��Һǡ����ɫ���������SO2�ĺ���Ϊ______��g/cm3����
��4��ʵ��������������______��
��Ҫ�ⶨ�����п���������ĺ�����g/L��������Ҫ�����������______
��5����ͬѧ����ͬ���ķ�������������SO2�ĺ���������õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ���ǣ�������Һ���ơ���������ȡ�����ֶ���������______��
�⣺��1������200mL0.1mol?L-1����KMnO4��Һ������ʵ����û��200mL����ƿ��Ӧ��250mL����ƿ���ƣ�����������ƽ����������ع��壬���ձ��м�ˮ�ܽⲢ�ò��������裬��ȴ���ò�����ת��������ƿ�У�����������������������ˮ����Һ��̶���1��2cmʱ���ý�ͷ�ιܵμ����̶��ߣ�
�ʴ�Ϊ����ƽ���ձ���250mL����ƿ����ͷ�ιܣ������������
��2��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ�
��Ӧ�ķ���ʽΪ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
�ʴ�Ϊ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
��3��������ص����ʵ���Ϊ0.2L��0.1moL/L=0.02moL��
���ݷ�Ӧ����ʽ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
��֪atml�����к���SO2�����ʵ���Ϊ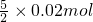 =0.05mol��
=0.05mol��
����Ϊ0.05mol��64g/moL=3.2g��
���Կ�����SO2�ĺ���Ϊ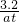 g/cm3��
g/cm3��
�ʴ�Ϊ��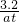 ��
��
��4�������������ԣ������������еĿ�������ֹ������������������ܻ�������Ը��������Һ�У�ʹ��������������Ҫ�ⶨ�����п���������ĺ�����g/L��������Ҫ���������������ǰ�����������������ʢ����������������
�ʴ�Ϊ����ֹ������������������ܻ�������Ը��������Һ�У�ʹ����������������ǰ�����������������ʢ������������������װ���ñ�ű�ʾ�ش𣬻�����������������ʢ��������������������ֵ����
��5�����õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�˵����������δ�����Ը��������Һ��ַ�Ӧ������ԭ�����������ʹ��죬
�ʴ�Ϊ��ͨ���������ʹ��죬��������δ�����Ը��������Һ��ַ�Ӧ���Ѿ����ų���
��������1����������200mL0.1mol?L-1����KMnO4��Һʱ����Ҫ�IJ��������ÿһ����������Ҫ������������
��2��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ��Դ���д���ӷ���ʽ��
��3�����ݷ�Ӧ�����ӷ���ʽ���㣻
��4�������������ԣ������������еĿ�����
��5�����õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�˵����������δ�����Ը��������Һ��ַ�Ӧ��
���������⿼�����ʵ���ɺͺ����IJⶨ����Ŀ���ѣ�����ע��ʵ���ԭ���Ͳ���������ע�����
�ʴ�Ϊ����ƽ���ձ���250mL����ƿ����ͷ�ιܣ������������
��2��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ�
��Ӧ�ķ���ʽΪ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
�ʴ�Ϊ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
��3��������ص����ʵ���Ϊ0.2L��0.1moL/L=0.02moL��
���ݷ�Ӧ����ʽ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
��֪atml�����к���SO2�����ʵ���Ϊ
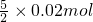 =0.05mol��
=0.05mol������Ϊ0.05mol��64g/moL=3.2g��
���Կ�����SO2�ĺ���Ϊ
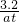 g/cm3��
g/cm3���ʴ�Ϊ��
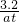 ��
����4�������������ԣ������������еĿ�������ֹ������������������ܻ�������Ը��������Һ�У�ʹ��������������Ҫ�ⶨ�����п���������ĺ�����g/L��������Ҫ���������������ǰ�����������������ʢ����������������
�ʴ�Ϊ����ֹ������������������ܻ�������Ը��������Һ�У�ʹ����������������ǰ�����������������ʢ������������������װ���ñ�ű�ʾ�ش𣬻�����������������ʢ��������������������ֵ����
��5�����õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�˵����������δ�����Ը��������Һ��ַ�Ӧ������ԭ�����������ʹ��죬
�ʴ�Ϊ��ͨ���������ʹ��죬��������δ�����Ը��������Һ��ַ�Ӧ���Ѿ����ų���
��������1����������200mL0.1mol?L-1����KMnO4��Һʱ����Ҫ�IJ��������ÿһ����������Ҫ������������
��2��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ��Դ���д���ӷ���ʽ��
��3�����ݷ�Ӧ�����ӷ���ʽ���㣻
��4�������������ԣ������������еĿ�����
��5�����õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�˵����������δ�����Ը��������Һ��ַ�Ӧ��
���������⿼�����ʵ���ɺͺ����IJⶨ����Ŀ���ѣ�����ע��ʵ���ԭ���Ͳ���������ע�����

��ϰ��ϵ�д�
�����Ŀ

 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ������� ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ���������



 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ������� ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ��������� ��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�
��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�