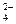��Ŀ����
һƿ����������Һ�����ܺ������������е�һ�ֻ��֣�Fe2+��Fe3+��Ba2+��Al3+��NH4+��SO42-�� HCO3-��NO3-��Cl-ȡ��Һ��������ʵ�飺
����Һ������ɫʯ����ֽ�ϣ���ֽ�ʺ�ɫ��
��ȡ������ҺŨ�������ͭƬ��Ũ���Ṳ�ȣ��к���ɫ�������ɡ�
��ȡ������Һ�������������ữ���Ȼ�����Һ��������ɫ������
���������еij������˳�����Һ�м�����������Һ�����ɰ�ɫ����
����ȡԭ��Һ����μ�������������Һ���������ȿ������ɳ�������֮�ó��������� �⣬���ʺ��ɫ��
��������ʵ�������ƶϣ�
��1����Һ�п϶����ڵ������� ��
��2����Һ�п϶������ڵ������� ��
��3����Һ�в���ȷ���Ƿ���ڵ������� ��
��4��������ʵ��������θĽ�������ȷ����3���е������Ƿ����
����Һ������ɫʯ����ֽ�ϣ���ֽ�ʺ�ɫ��
��ȡ������ҺŨ�������ͭƬ��Ũ���Ṳ�ȣ��к���ɫ�������ɡ�
��ȡ������Һ�������������ữ���Ȼ�����Һ��������ɫ������
���������еij������˳�����Һ�м�����������Һ�����ɰ�ɫ����
����ȡԭ��Һ����μ�������������Һ���������ȿ������ɳ�������֮�ó��������� �⣬���ʺ��ɫ��
��������ʵ�������ƶϣ�
��1����Һ�п϶����ڵ������� ��
��2����Һ�п϶������ڵ������� ��
��3����Һ�в���ȷ���Ƿ���ڵ������� ��
��4��������ʵ��������θĽ�������ȷ����3���е������Ƿ����
��1����Һ�п϶����ڵ������� Fe3+��Al3+��NO3����SO42�� ��
��2����Һ�п϶������ڵ�������HCO3����Ba2+��Fe2+ ��
��3����Һ�в���ȷ���Ƿ���ڵ������� NH4+��Cl�� �����϶Ե�����ÿ��1�֣���
��4���Ѣ۲��е��Ȼ����������ᱵ���ٲ����ܾͿ���ȷ��Cl���Ĵ��ڣ��Բ����ݵ���Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�Ƿ��������ȷ��NH4+��������ȡ����ԭ��Һ���Թ��в�����������NaOH��Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�Ƿ��������ȷ��NH4+��������������Ҳ�ɣ� ��4�֣�
��2����Һ�п϶������ڵ�������HCO3����Ba2+��Fe2+ ��
��3����Һ�в���ȷ���Ƿ���ڵ������� NH4+��Cl�� �����϶Ե�����ÿ��1�֣���
��4���Ѣ۲��е��Ȼ����������ᱵ���ٲ����ܾͿ���ȷ��Cl���Ĵ��ڣ��Բ����ݵ���Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�Ƿ��������ȷ��NH4+��������ȡ����ԭ��Һ���Թ��в�����������NaOH��Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�Ƿ��������ȷ��NH4+��������������Ҳ�ɣ� ��4�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��Cu
��Cu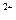 ��Br
��Br ��SO
��SO
 I
I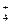 ��Fe
��Fe ��SO
��SO ����Һ��Na
����Һ��Na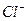 ��
��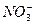 ��
��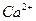 ��
��
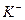 ��
�� ��
��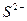
 ��
�� ��
��
 ��
�� ��
�� ��
��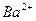
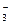 ��SO
��SO