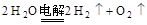��Ŀ����
��Դ�ɻ���Ϊһ����Դ�Ͷ�����Դ����Ȼ�����ֳ���ʽ�ṩ����Դ��Ϊһ����Դ��������������Դ�����ȡ����Դ��Ϊ������Դ��������һ�ָ�Ч��û����Ⱦ�Ķ�����Դ������������Ȼ���д������ڵ�ˮ����ȡ��2H2O��1���T�T2H2(g)+O2(g)��H=517.6kJ/mol�����������������ش����⣺
����������ȷ���ǣ�����
A�������Ƕ�����Դ���������������������������������������� B��ˮ���Ƕ�����Դ
C����Ȼ����һ����Դ������������������������������������ D����¯����һ����Դ
��֪��CH4(g)+2O2(g)�T�T2H2O��1��+CO2(g)����H=-890.3kJ/mol��1g������1g CH4�ֱ�ȼ�պų�������֮��ԼΪ������
A��1�U3.4���������������� B��1�U1.7���������������� C��2.3�U1���������������� D��4.6�U1
������ˮ��ȡ������Դ�����������о�������ȷ���ǣ�����
A������H2O����������ǿ�ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B���跨��̫����۽����������£�ʹˮ�ֽ����H2
C��Ѱ�Ҹ�Ч������ʹˮ�ֽ����H2��ͬʱ�ͷ�����
D��Ѱ�����⻯ѧ���ʣ����ڿ���������Դ���Էֽ�ˮ��ȡH2
�𰸣�AC;C;BD

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ