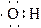��Ŀ����
��ѧ������ѧϰ��ѧ����Ҫ���ߡ�����������ʾ���ʱ仯�Ļ�ѧ��������ȷ���ǣ� ��
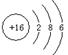
| A��K37ClO3��ŨHCl�ڼ�������������Cl2�Ļ�ѧ����ʽ: K37ClO3+6HCl=K37Cl+3Cl2��+3H2O |
B����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ: H2��g��+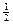 O2��g��=H2O��g������H= -241��8KJ/mol O2��g��=H2O��g������H= -241��8KJ/mol |
| C����1~2ml FeCl3������Һ���뵽20ml��ˮ�������������ӷ���ʽ: Fe3++3H2O 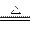 Fe��OH�� 3�����壩+3H+ Fe��OH�� 3�����壩+3H+ |
| D����Ba��OH�� 2��Һ�е�����������������: Ba2++OH-+H++SO42-=BaSO4��+ H2O |
C
��

��ϰ��ϵ�д�
�����Ŀ

 HCOO- +NH4++2Ag��+3NH3+H2O
HCOO- +NH4++2Ag��+3NH3+H2O
 Br
Br �����˻��Ϸ���ǹ���á���ҩ������Ҫ�ɷ�������غͺ��ף�ײ��ʱ�����Ļ�ѧ��ӦΪ��5KC1O3+6P
�����˻��Ϸ���ǹ���á���ҩ������Ҫ�ɷ�������غͺ��ף�ײ��ʱ�����Ļ�ѧ��ӦΪ��5KC1O3+6P