��Ŀ����
��2009?������[��ѧ--�л���ѧ����]
�л���A�dz��õ�ʳ���Ϳ�������������ʽΪC10H12O5���ɷ�������ת����
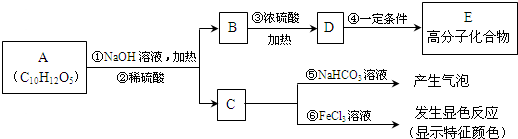
��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ��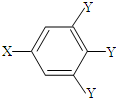 �����У�-X��-Y��Ϊ�����ţ���
�����У�-X��-Y��Ϊ�����ţ���
��ش��������⣺
��1������ϵͳ��������B������Ϊ
��2��������-X������Ϊ
 ��
��
��3��A�Ľṹ��ʽΪ
 ��
��
��4����Ӧ�ݵĻ�ѧ����ʽΪ +NaHCO3��
+NaHCO3�� +H2O+CO2��
+H2O+CO2�� +NaHCO3��
+NaHCO3�� +H2O+CO2����
+H2O+CO2����
��5��C�ж���ͬ���칹�壬д������2�ַ�������Ҫ���ͬ���칹��Ľṹ��ʽ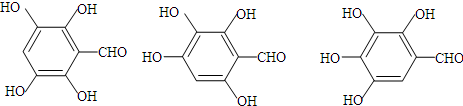 ��д������3���ṹ��ʽ�е�����2�����ɣ�
��д������3���ṹ��ʽ�е�����2�����ɣ� ��д������3���ṹ��ʽ�е�����2�����ɣ���
��д������3���ṹ��ʽ�е�����2�����ɣ���
i�����б��� ii���ܷ���������Ӧ iii�����ܷ���ˮ�ⷴӦ
��6���ӷ��ӽṹ�Ͽ���A���п��������õ���Ҫԭ����
a�����б��� b�������ʻ� c�����з��ǻ���
�л���A�dz��õ�ʳ���Ϳ�������������ʽΪC10H12O5���ɷ�������ת����

��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ��
 �����У�-X��-Y��Ϊ�����ţ���
�����У�-X��-Y��Ϊ�����ţ�����ش��������⣺
��1������ϵͳ��������B������Ϊ
1-����
1-����
����2��������-X������Ϊ
�Ȼ�
�Ȼ�
���߾���E������Ϊ

��3��A�Ľṹ��ʽΪ


��4����Ӧ�ݵĻ�ѧ����ʽΪ
 +NaHCO3��
+NaHCO3�� +H2O+CO2��
+H2O+CO2�� +NaHCO3��
+NaHCO3�� +H2O+CO2��
+H2O+CO2����5��C�ж���ͬ���칹�壬д������2�ַ�������Ҫ���ͬ���칹��Ľṹ��ʽ
 ��д������3���ṹ��ʽ�е�����2�����ɣ�
��д������3���ṹ��ʽ�е�����2�����ɣ� ��д������3���ṹ��ʽ�е�����2�����ɣ�
��д������3���ṹ��ʽ�е�����2�����ɣ�i�����б��� ii���ܷ���������Ӧ iii�����ܷ���ˮ�ⷴӦ
��6���ӷ��ӽṹ�Ͽ���A���п��������õ���Ҫԭ����
c
c
������ţ���a�����б��� b�������ʻ� c�����з��ǻ���
����������B����Է��������ͺͽṹ�Լ��ܷ�����ȥ��Ӧ�õ�ϩ�������ʿ��Եó�B�Ľṹ��ʽ������ȷ��DΪB��ȥ�IJ��EΪϩ���Ӿ۵IJ������ȷ�����ڣ��ܺ�̼�����Ʒ�����Ӧ���ɶ�����̼��һ���Ǻ����Ȼ��Ļ������ʹ�Ȼ���������ɫ��Ӧ��һ���Ǻ��з��ǻ������ʣ����ݽṹ��ȷ��C��A��B��C����������Ӧ�IJ���������Եó�A�Ľṹ��ʽ��
����⣺B����Է�������Ϊ60��������ֻ��һ����������Ũ����������·�����ȥ��Ӧ����BΪ1-��������CH3CH2CH2OH��������ȥ��Ӧ�õ���ϩ����DΪCH3CH�TCH2���߷��ӻ�����EΪ�۱�ϩ��
C�ܺ�̼���Ʒ�����Ӧ���ɶ�����̼����һ�������Ȼ����ܺ��Ȼ�����Һ������ɫ��Ӧ��һ�����з��ǻ�������cΪ�� ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ��
��
��1��B������Ϊ1-�������ʴ�Ϊ��1-������
��2��C�ܺ�̼�����Ʒ�����Ӧ���ɶ�����̼����һ�������Ȼ����ܺ��Ȼ�����Һ������ɫ��Ӧ��һ�����з��ǻ�����ΪC��A��ˮ��IJ�������Ȼ�ֻ��һ������-XΪ�Ȼ���DΪCH3CH�TCH2���߷��ӻ�����EΪ�۱�ϩ������E�ĵ���Ϊ�۱�ϩ������Ϊ�� ���ʴ�Ϊ���Ȼ���
���ʴ�Ϊ���Ȼ��� ��
��
��3��BΪ1-��������CH3CH2CH2OH��cΪ�� ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4�������Ȼ������ʿ��Ժ�̼�����Ʒ�����Ӧ���ɶ�����̼������ ��̼�����Ʒ�����Ӧ��ԭ������ʽΪ��
��̼�����Ʒ�����Ӧ��ԭ������ʽΪ�� +NaHCO3��
+NaHCO3�� +H2O+CO2�����ʴ�Ϊ��
+H2O+CO2�����ʴ�Ϊ�� +NaHCO3��
+NaHCO3�� +H2O+CO2����
+H2O+CO2����
��5���ܷ���������Ӧ��������һ������ȩ����������ǻ�ȩ�����ʻ�Ϊͬ���칹�壬���Խ��Ȼ��������ǻ���ȩ������ϣ����Է���Ҫ��Ľṹ��ʽ���£�
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��д������3���ṹ��ʽ�е�����2�����ɣ���
��д������3���ṹ��ʽ�е�����2�����ɣ���
��6��A���п��������õ���Ҫԭ���Ƿ������з��ǻ������ݱ����ڿ������ױ�����Ϊ�ۺ�ɫ��ȷ�����ɣ��ʴ�Ϊ��c��
C�ܺ�̼���Ʒ�����Ӧ���ɶ�����̼����һ�������Ȼ����ܺ��Ȼ�����Һ������ɫ��Ӧ��һ�����з��ǻ�������cΪ��
 ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ��
����1��B������Ϊ1-�������ʴ�Ϊ��1-������
��2��C�ܺ�̼�����Ʒ�����Ӧ���ɶ�����̼����һ�������Ȼ����ܺ��Ȼ�����Һ������ɫ��Ӧ��һ�����з��ǻ�����ΪC��A��ˮ��IJ�������Ȼ�ֻ��һ������-XΪ�Ȼ���DΪCH3CH�TCH2���߷��ӻ�����EΪ�۱�ϩ������E�ĵ���Ϊ�۱�ϩ������Ϊ��
 ���ʴ�Ϊ���Ȼ���
���ʴ�Ϊ���Ȼ��� ��
����3��BΪ1-��������CH3CH2CH2OH��cΪ��
 ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4�������Ȼ������ʿ��Ժ�̼�����Ʒ�����Ӧ���ɶ�����̼������
 ��̼�����Ʒ�����Ӧ��ԭ������ʽΪ��
��̼�����Ʒ�����Ӧ��ԭ������ʽΪ�� +NaHCO3��
+NaHCO3�� +H2O+CO2�����ʴ�Ϊ��
+H2O+CO2�����ʴ�Ϊ�� +NaHCO3��
+NaHCO3�� +H2O+CO2����
+H2O+CO2������5���ܷ���������Ӧ��������һ������ȩ����������ǻ�ȩ�����ʻ�Ϊͬ���칹�壬���Խ��Ȼ��������ǻ���ȩ������ϣ����Է���Ҫ��Ľṹ��ʽ���£�
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��д������3���ṹ��ʽ�е�����2�����ɣ���
��д������3���ṹ��ʽ�е�����2�����ɣ�����6��A���п��������õ���Ҫԭ���Ƿ������з��ǻ������ݱ����ڿ������ױ�����Ϊ�ۺ�ɫ��ȷ�����ɣ��ʴ�Ϊ��c��
������������һ���й��л�������Ľṹ�����ʵ��ۺ��ƶ��⣬����ѧ�������ͽ��������������ۺ��Ժ�ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
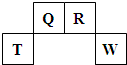 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�

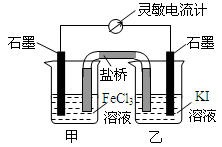 ��2009?�����������ʺϵ�����������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ�����ͼ��ʾ��ԭ��أ������жϲ���ȷ���ǣ�������
��2009?�����������ʺϵ�����������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ�����ͼ��ʾ��ԭ��أ������жϲ���ȷ���ǣ�������