��Ŀ����
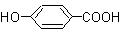
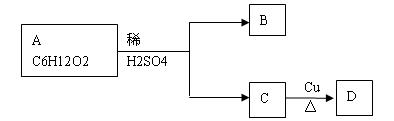
��12�֣�PMA��ij���˹��ϳɵ��л��߷��ӻ�����ж�����;������Դ��ʯ���ѽ����ļ�Ϊԭ�ϣ�ͨ������;���ϳ�PMA��

��֪�����������Է�������Ϊ90��̼����������Ϊ40%�������������Ϊ6.67%������Ϊ����������������⣺
��1���Ľṹ��ʽΪ ��
��2��������з�Ӧ����ʽ��ָ����Ӧ����
��Ӧ�� �� ��
��Ӧ�� �� ��
��3���ݷ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��4�����ж���ͬ���칹�壬д��һ��ͬʱ����ٲ��ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ������ij����ͬ�������Ҳ�����ͬһ��̼ԭ���ϡ�����������ͬ���칹��Ľṹ��ʽ�� ��

��֪�����������Է�������Ϊ90��̼����������Ϊ40%�������������Ϊ6.67%������Ϊ����������������⣺
��1���Ľṹ��ʽΪ ��
��2��������з�Ӧ����ʽ��ָ����Ӧ����
��Ӧ�� �� ��
��Ӧ�� �� ��
��3���ݷ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��4�����ж���ͬ���칹�壬д��һ��ͬʱ����ٲ��ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ������ij����ͬ�������Ҳ�����ͬһ��̼ԭ���ϡ�����������ͬ���칹��Ľṹ��ʽ�� ��
��12�֣���ע���⣬����ÿ��2�֣���1��CH2��CHCH3��
��2����CH2BrCHBrCH3��2H2O CH2OHCHOHCH3��2HBr ��д�����������Ʒ�ӦҲ���֣���ˮ�ⷴӦ��ȡ����Ӧ��1�֣���
CH2OHCHOHCH3��2HBr ��д�����������Ʒ�ӦҲ���֣���ˮ�ⷴӦ��ȡ����Ӧ��1�֣���
�� ���Ӿ۷�Ӧ��ۺϷ�Ӧ��1�֣���
���Ӿ۷�Ӧ��ۺϷ�Ӧ��1�֣���
��3��
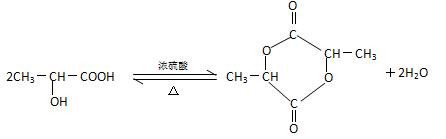
��4�� CH2OH��CHOH��CHO��
��2����CH2BrCHBrCH3��2H2O
 CH2OHCHOHCH3��2HBr ��д�����������Ʒ�ӦҲ���֣���ˮ�ⷴӦ��ȡ����Ӧ��1�֣���
CH2OHCHOHCH3��2HBr ��д�����������Ʒ�ӦҲ���֣���ˮ�ⷴӦ��ȡ����Ӧ��1�֣�����
 ���Ӿ۷�Ӧ��ۺϷ�Ӧ��1�֣���
���Ӿ۷�Ӧ��ۺϷ�Ӧ��1�֣�����3��

��4�� CH2OH��CHOH��CHO��
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

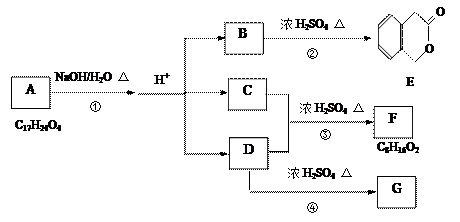
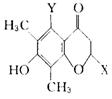
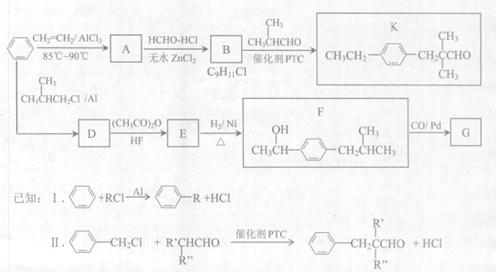
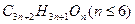 ����ž��صķ�Ϊ ��
����ž��صķ�Ϊ �� ����ž��ط�����һY������Ϊ ��
����ž��ط�����һY������Ϊ ��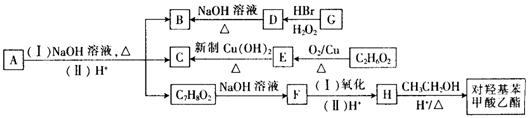
 H
H CH2+HBr
CH2+HBr CH3CH2CH2Br
CH3CH2CH2Br B�Ļ�ѧ����ʽΪ
B�Ļ�ѧ����ʽΪ  ��
�� ������ �֣����ǻ������������������⣩��
������ �֣����ǻ������������������⣩��