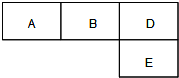
��Ŀ����
 ��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ������
��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ�����������壬NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ�A��B��D��E�ֱ����ұ������ڱ���һ���֣�ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37���Իش�
��1��A��B��D��E����Ԫ�طֱ���MԪ���γɵļ������У��е���ߵ���
H2O
H2O
���ѧʽ������2��M��D��E��N�γɵļ����ӵİ뾶�ɴ�С�Ĺ�ϵ�ǣ�д���ӷ��ţ�
S2-��O2-��Na+��H+
S2-��O2-��Na+��H+
����3��A��B��D��M����ɶ���18���ӷ��ӣ���д��1�־���18���ӵ��л���ķ���ʽ
CH3OH
CH3OH
����4����A��D��N����Ԫ����ɵ������ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��
CO32-+H2O?HCO3-+OH-
CO32-+H2O?HCO3-+OH-
����5��Ԫ��B���⻯����Ԫ��D������һ�������·����û���Ӧ���ڸ÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ
3��4
3��4
����6������M��D��N��E����Ԫ�ع��ɵĻ������ˮ��Һ�е���������Ba��OH��2��Һ��д�����ܷ��������з�Ӧ�����ӷ���ʽ
2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-
2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-
��������MԪ�صĵ�������Ȼ����������壬MΪH��NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ���NӦΪ�������ڵڢ�A���Na�����ͼ�е�λ�ã���A��ԭ������Ϊx����BΪx+1��cΪx+2��EΪx+10�����ǵ�ԭ������֮��Ϊ37������x+x+1+x+2+x+10=37��x=6����AΪC��BΪN��DΪO��EΪS��Ȼ����Ԫ�ؼ��䵥�ʡ�����������������
����⣺MԪ�صĵ�������Ȼ����������壬MΪH��NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ���NӦΪ�������ڵڢ�A���Na�����ͼ�е�λ�ã���A��ԭ������Ϊx����BΪx+1��cΪx+2��EΪx+10�����ǵ�ԭ������֮��Ϊ37������x+x+1+x+2+x+10=37��x=6����AΪC��BΪN��DΪO��EΪS��
��1��A��B��D��E����Ԫ�طֱ���MԪ���γɵļ������У�B��M��C��M�ķ����к����������H2O�ķе���ʴ�Ϊ��H2O��
��2�����Ӳ���Խ�࣬���Ӱ뾶Խ���������������Ӿ�����ͬ�ĵ����Ų�������ԭ������С���Ӱ뾶�������Ӱ뾶ΪS2-��O2-��Na+��H+���ʴ�Ϊ��S2-��O2-��Na+��H+��
��3����ԭ�ӵ���������֪������18���ӵ��л���ķ���ʽΪCH3OH��CH3CH3�ȣ��ʴ�Ϊ��CH3OH��
��4����A��D��N����Ԫ����ɵ������ˮ��Һ�ʼ��ԣ�������Ϊ̼���ƣ�̼�������ˮ��ʹ��Һ�Լ��ԣ����ӷ�ӦΪCO32-+H2O?HCO3-+OH-���ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��5��Ԫ��B���⻯����Ԫ��D������һ�������·����û���Ӧ���÷�ӦΪ4NH3+3O2
2N2+6H2O��NԪ�صĻ��ϼ����ߣ�OԪ�صĻ��ϼ۽��ͣ�������Ϊ������������Ϊ��ԭ�������Ը÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ3��4���ʴ�Ϊ��3��4��
��6����M��D��N��E����Ԫ�ع��ɵĻ�����ΪNaHSO4��NaHSO3������������Ba��OH��2��Һ��Ba��OH��2��ȫ��Ӧ�����������ӷ�ӦΪ2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-���ʴ�Ϊ��2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-��
��1��A��B��D��E����Ԫ�طֱ���MԪ���γɵļ������У�B��M��C��M�ķ����к����������H2O�ķе���ʴ�Ϊ��H2O��
��2�����Ӳ���Խ�࣬���Ӱ뾶Խ���������������Ӿ�����ͬ�ĵ����Ų�������ԭ������С���Ӱ뾶�������Ӱ뾶ΪS2-��O2-��Na+��H+���ʴ�Ϊ��S2-��O2-��Na+��H+��
��3����ԭ�ӵ���������֪������18���ӵ��л���ķ���ʽΪCH3OH��CH3CH3�ȣ��ʴ�Ϊ��CH3OH��
��4����A��D��N����Ԫ����ɵ������ˮ��Һ�ʼ��ԣ�������Ϊ̼���ƣ�̼�������ˮ��ʹ��Һ�Լ��ԣ����ӷ�ӦΪCO32-+H2O?HCO3-+OH-���ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��5��Ԫ��B���⻯����Ԫ��D������һ�������·����û���Ӧ���÷�ӦΪ4NH3+3O2
| ||
��6����M��D��N��E����Ԫ�ع��ɵĻ�����ΪNaHSO4��NaHSO3������������Ba��OH��2��Һ��Ba��OH��2��ȫ��Ӧ�����������ӷ�ӦΪ2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-���ʴ�Ϊ��2H++2OH-+SO42-+Ba2+�TBaSO4��+2H2O��2HSO3-+2OH-+Ba2+�TBaSO3��+2H2O+SO32-��
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ԫ�ص��ƶ��ǽ����Ĺؼ�������Ϥˮ�⡢���ӷ�Ӧ����ѧ��Ӧ��֪ʶ������ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ���5������6��Ϊ�����״��㣮

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
�����Ŀ
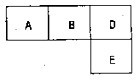 ��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ����������壬NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ�A��B��D��E�ֱ��ڱ������ڱ���һ���֣�ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37���Իش�
��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ����������壬NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ�A��B��D��E�ֱ��ڱ������ڱ���һ���֣�ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37���Իش�