��Ŀ����
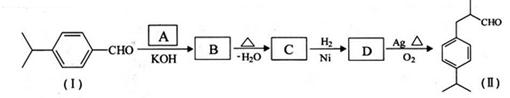
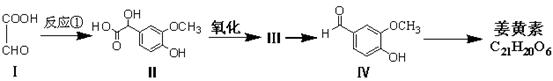
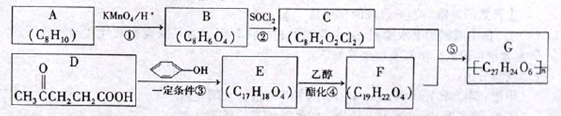
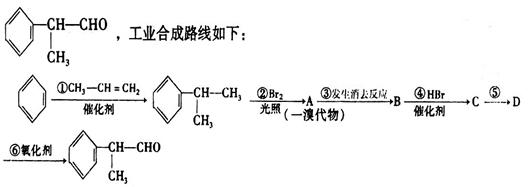
�ö���ȩ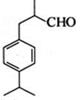 ��һ����Ҫ�����ϡ����л���IΪԭ�Ͽ��Ժϳ��ö���ȩ����ϳ�·����ͼ��ʾ��
��һ����Ҫ�����ϡ����л���IΪԭ�Ͽ��Ժϳ��ö���ȩ����ϳ�·����ͼ��ʾ��
��֪��
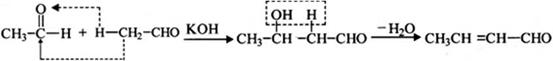
�Իش��������⣺
��1���ö���ȩ�ķ���ʽ�� ������A�Ľṹ��ʽ�� ��
��2������C�к��е�̼̼˫�������Լ��� ����ѡ����
��3��C��D�ķ�Ӧ�����ǣ� ���䷴Ӧ�Ļ�ѧ����ʽΪ��
(ע����Ӧ��������
��4���ö���ȩ�еĺ����������ױ����������ɻ�����W��������W�Ľṹ��ʽ�� ��
��5���л���I�ж���ͬ���칹�壬����һ��ͬ���칹����ʹFeCl3��Һ����ɫ���ṹ�в�����CH3���ұ�����ֻ��������Ϊ��λ��ȡ����������ܵĽṹ��ʽΪ ��д��һ�֣���
 ��һ����Ҫ�����ϡ����л���IΪԭ�Ͽ��Ժϳ��ö���ȩ����ϳ�·����ͼ��ʾ��
��һ����Ҫ�����ϡ����л���IΪԭ�Ͽ��Ժϳ��ö���ȩ����ϳ�·����ͼ��ʾ��
��֪��
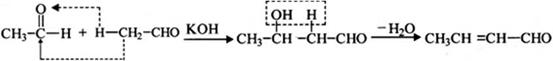
�Իش��������⣺
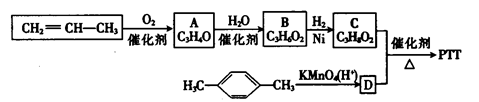
��1���ö���ȩ�ķ���ʽ�� ������A�Ľṹ��ʽ�� ��
��2������C�к��е�̼̼˫�������Լ��� ����ѡ����
| A������KMnO4��Һ | B�����Ʊ�Cu(OH)2����Һ | C����ˮ | D�����CCl4��Һ |
(ע����Ӧ��������
��4���ö���ȩ�еĺ����������ױ����������ɻ�����W��������W�Ľṹ��ʽ�� ��
��5���л���I�ж���ͬ���칹�壬����һ��ͬ���칹����ʹFeCl3��Һ����ɫ���ṹ�в�����CH3���ұ�����ֻ��������Ϊ��λ��ȡ����������ܵĽṹ��ʽΪ ��д��һ�֣���
��16�֣���1��C13H18O��2�֣� CH3CH2CHO��2�֣�
��2��D��2�֣�
��3����ԭ��Ӧ��ӳɷ�Ӧ��2�֣�
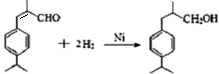 ��3�֣�
��3�֣�
��4��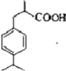 ��2�֣�
��2�֣�
��5��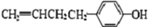 ��
��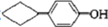 ��3�֣�
��3�֣�
��2��D��2�֣�
��3����ԭ��Ӧ��ӳɷ�Ӧ��2�֣�
 ��3�֣�
��3�֣���4��
 ��2�֣�
��2�֣���5��
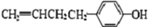 ��
�� ��3�֣�
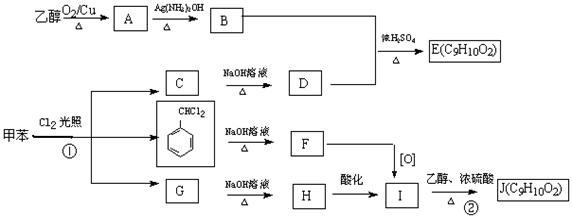
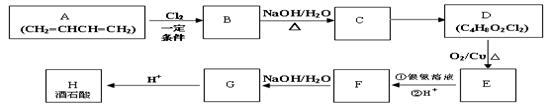
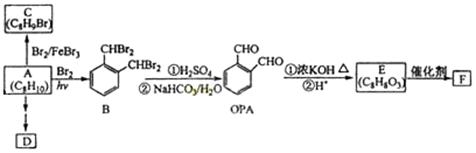
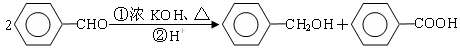
��3�֣������������1���۲��ö���ȩ�Ľṹ��ʽ����һ������C��H��O�ĸ������ɵ������ʽΪC13H18O�����ö���ȩ�ĺϳ�·�����ƣ�D����ö���ȩ�ķ�Ӧ�Ǵ���Ag��Cu����������������������Ӧ��ֻ�д��ǻ���Ϊȩ���������л���������ṹ��ͬ���ɴ˿����Ƶ�D����1��������λ�ڱ��������λ�õ�2��ȡ�����ֱ���(CH3)2CH������CH2CH(CH3)CH2OH����D�Ľṹ��ʽΪ(CH3)2CH��C6H4��CH2CH(CH3)CH2OH��C��ΪD�Ǽӳɷ�Ӧ����B���C�Ǵ�����ȥ��Ӧ��������֪��Ϣ��֪������I��ΪA��I��ȩ����A��ȩ����̼ԭ���ϵ�C��H��֮��ļӳɷ�Ӧ���ɴ˿���ȷ��A�Ľṹ��ʽ��CH3CH2CHO��B�Ľṹ��ʽΪ(CH3)2CH��C6H4��CHOHCH(CH3)CHO��C�Ľṹ��ʽΪ(CH3)2CH��C6H4��CH=C(CH3)CHO����2��C����1��������λ�ڱ��������λ�õ�2��ȡ�����ֱ���(CH3)2CH������CH=C(CH3)CHO������������̼̼˫����ȩ�����뱽��ֱ��������̼ԭ����C��H�����ܱ����Ը��������Һ���������ʹ���Ը��������Һ��ɫ��ԭ��һ����������̼̼˫������Aѡ���������������ͭ���ڼ���C�к��е�ȩ��������ʱ����ש��ɫ������˵��C�к���ȩ�����������ڼ���̼̼˫������Bѡ�����ȩ�����Ա���ˮ�������Ȼ���̼̼˫���������巢���ӳɷ�Ӧ�����ʹ��ˮ��ɫ��ԭ��һ����C�к���̼̼˫������Cѡ�����������Ȼ�̼��Һֻ����̼̼˫�������ӳɷ�Ӧ����������ȩ������Ϊ�ܼ�����ˮ�����ʹ������Ȼ�̼��Һ��ɫ��˵��C�к���̼̼˫������Dѡ����ȷ����3��C��������̼̼˫����ȩ�����������������ӳɷ�Ӧ��ԭ��Ӧ������1��̼̼˫�������1���������Ӽӳɣ�1��ȩ�������1���������Ӽӳɣ���1molC��2molH2���������·����ӳɷ�Ӧ������1molD���ɴ˿�����д�÷�Ӧ�Ļ�ѧ����ʽ���ö���ȩ�Ĺ�����ֻ��ȩ����ȩ���Ǻ��������ţ����Ա�����Ϊ�Ȼ�����W ����1��������λ�ڱ��������λ�õ�2��ȡ�����ֱ���(CH3)2CH������CH2CH(CH3)COOH���ɴ˿�����дW�Ľṹ��ʽ����5���۲�I�Ľṹ��ʽ����һ������C��H��Oԭ�Ӹ������ɵ������ʽΪC10H12O�����������I��ͬ���칹�庬��1�����ǻ���λ�ڱ������ǻ���λ��ȡ���������ǡ�CH2CH2CH=CH2�������ɴ˿���ȷ����ͬ���칹��Ľṹ��ʽ��

��ϰ��ϵ�д�
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
�����Ŀ
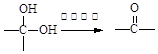

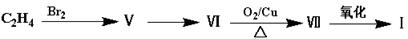
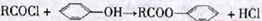

 ��
��