��Ŀ����
Ϊ�ⶨijþ���Ͻ���Ʒ�����ĺ���������������ʵ�飺ȡһ�����Ͻ𣬼���100 mL 0.3 mol��L��1ϡ���ᣬ�Ͻ���ȫ�ܽ⣬�����������ڱ�״�������Ϊ560 mL���ټ���0.2 mol��L��1NaOH��Һ����������ǡ�ò��ٱ仯����ȥ350 mL NaOH��Һ������ȡ��Ʒ���������ʵ���Ϊ(����)
A��0.005 mol�� B��0.01 mol�� C��0.025 mol�� D��0.03 mol
���𰸡�
B
��������������ҺΪNa2SO4��NaAlO2�Ļ��Һ����Ԫ���غ��֪Na��Ϊ0.07 mol��SO42��Ϊ0.03mol����֪AlO2��Ϊ0.01 mol��Ҳ����˵ԭ�Ͻ�����Ϊ0.01 mol

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ
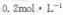 NaOH��Һ�������������ٱ仯����ȥ350mLNaOH��Һ������ȡ��Ʒ���������ʵ���Ϊ �� )
NaOH��Һ�������������ٱ仯����ȥ350mLNaOH��Һ������ȡ��Ʒ���������ʵ���Ϊ �� )