��Ŀ����
ij�����ų�����ˮ�к��д�����Fe2+��Zn2+��Hg2+���ӣ�������ijѧϰС���ͬѧ��Ƶij�ȥ�����������ӣ������̷���𩷯��ZnSO4?7H2O������̽����������֪��KSP��FeS��=6.3��10-18mol2?L-2��KSP��HgS��=6.4��10-53mol2?L-2KSP��ZnS��=1.6��10-24mol2?L-2
ҩƷ��NaOH��Һ��Na2S��Һ��ϡ���ᡢ���� ϡ���ᡢϡ����
[ʵ�鷽��]
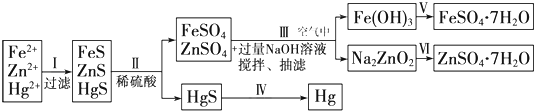
[����̽��]
��1��������м���Na2S��Һʱ�����ֽ��������γɳ������Ⱥ�˳��Ϊ
��2���������FeS�ܽ�����ӷ���ʽ��
��3����������漰��Ӧ�����ӷ���ʽ Zn2++4OH-�TZnO
2- 2 |
��4�������Ͳ������������ط�Ӧ��˵��Zn��OH��2����
��5����ʵ�ֲ�������������Լ�Ϊ
��6����������õķ����Ǽ��ȷ������ڿ����м���HgS ����Ⱦ��������ԭ�����
��������1������Na2S��Һʱ�����ֽ���������KspԽС������Խ���ȳ��ֳ������ݴ����ش�
��2�����������Ժ�����֮�䷢����Ӧ���������������������壻
��3���ڿ����У����������ױ����������������ӣ��ڼ��Ի����£��������������������
��4��������п���Ժ��������Ʒ�Ӧ������������п�����ʺ������������ƣ�
��5������������ˮ�⣬���ױ�������
��6���ڿ����м���HgSʱ���ᱻ�����е�������������SO2��Hg��
��2�����������Ժ�����֮�䷢����Ӧ���������������������壻
��3���ڿ����У����������ױ����������������ӣ��ڼ��Ի����£��������������������
��4��������п���Ժ��������Ʒ�Ӧ������������п�����ʺ������������ƣ�
��5������������ˮ�⣬���ױ�������
��6���ڿ����м���HgSʱ���ᱻ�����е�������������SO2��Hg��
����⣺��1������Na2S��Һʱ�����ֽ���������KspԽС������Խ���ȳ��ֳ��������Բ���������˳���ǣ�Hg2+��Zn2+��Fe2+���ʴ�Ϊ��Hg2+��Zn2+��Fe2+��
��2�����������Ժ�����֮�䷢����Ӧ���������������������壬����ǿ��������ԭ������FeS+2H+�TFe2++H2S�����ʴ�Ϊ��FeS+2H+�TFe2++H2S����
��3���ڿ����У����������ױ����������������ӣ��ڼ��Ի����£�������������������������Ļ�ѧ����ʽΪ��4Fe2++O2+8OH-+2H2O�T4Fe��OH��3����
�ʴ�Ϊ��4Fe2++O2+8OH-+2H2O�T4Fe��OH��3����
��4��������п���Ժ�������������ƫп���ƣ���Ӧ������������п�����ʺ������������ƣ�����������������ʴ�Ϊ�������������
��5��������������ȡ����������Ϊ�˷�ֹˮ�⣬���Լ������ᣬΪ��ֹ�����������Լ�����������ʴ�Ϊ��ϡ������ۣ�����������ֹFe2+ˮ�⣻
��6��HgS���ױ������е�������������SO2��Hg�����ɵ�SO2��Hg����Ⱦ�����ͻ������ʴ𰸣�HgS�������е�������������SO2�����ɵ�Hg����Ⱦ�����ͻ�����
��2�����������Ժ�����֮�䷢����Ӧ���������������������壬����ǿ��������ԭ������FeS+2H+�TFe2++H2S�����ʴ�Ϊ��FeS+2H+�TFe2++H2S����
��3���ڿ����У����������ױ����������������ӣ��ڼ��Ի����£�������������������������Ļ�ѧ����ʽΪ��4Fe2++O2+8OH-+2H2O�T4Fe��OH��3����
�ʴ�Ϊ��4Fe2++O2+8OH-+2H2O�T4Fe��OH��3����
��4��������п���Ժ�������������ƫп���ƣ���Ӧ������������п�����ʺ������������ƣ�����������������ʴ�Ϊ�������������
��5��������������ȡ����������Ϊ�˷�ֹˮ�⣬���Լ������ᣬΪ��ֹ�����������Լ�����������ʴ�Ϊ��ϡ������ۣ�����������ֹFe2+ˮ�⣻
��6��HgS���ױ������е�������������SO2��Hg�����ɵ�SO2��Hg����Ⱦ�����ͻ������ʴ𰸣�HgS�������е�������������SO2�����ɵ�Hg����Ⱦ�����ͻ�����
������������һ������Ԫ�غͻ�����֪ʶ�ۺϿ����⣬����ѧ�������ͽ��������������ۺ���ǿ���Ѷȴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ