��Ŀ����
����Ŀ����1������a.BaCl2 b.���ʯ c.NH4Cl d.Na2SO4 e.�ɱ� f.��Ƭ�������ʣ�������Ҫ��ش����ж��ѡ��ģ���ѡ��ѡ�����֣���
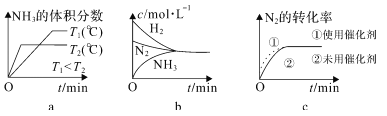
���ۻ�ʱ��Ҫ�ƻ����ۼ�����______�� �۵���ߵ���_______���۵���͵���_______��
���������ӻ��������________��ֻ�������Ӽ���������________���������ֻ�ѧ����������_________�����з��Ӽ�����������__________��
��2������ͼ�ش���������
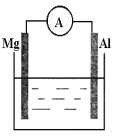
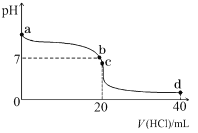
�����ձ�����ҺΪϡ���ᣬ��۲쵽��������___________��������ӦʽΪ___________��
�����ձ�����ҺΪ����������Һ����Ϊ_______����Mg��Al�����ܷ�Ӧ����ʽΪ__________��
���𰸡�b beacdacdefþ���ܽ⣬����������ð����������ָ�뷢��ƫתMg-2e-��Mg2+Al2Al+2NaOH+2H2O��2NaAlO2+3H2��
��������
(1)���ۻ�ʱ�ƻ����ۼ�˵���������д��ڹ��ۼ������ʯ����ԭ�Ӿ��壬���Ƚ��ʯ�ۻ��ƻ����ۼ������ʯ��ԭ�Ӿ��壬���۵���ߣ����Ӿ�����۵���ͣ��ҷ��Ӿ�����۵�������Է������������ȣ�������̼����Է�������С�ڵ⣬���Ըɱ����۵���ͣ��ʴ�Ϊ��b��b��e��
�����������ӹ��ɵ�Ϊ���ӻ�������������ӻ��������a��BaCl2��c��NH4Cl��d��Na2SO4��ֻ�����Ӽ���������a��BaCl2�����庬�����ֻ�ѧ������c��NH4Cl d��Na2SO4�����Ӿ�������������Ϊ���Ӽ������������з��Ӽ�����������e���ɱ� f����Ƭ���ʴ�Ϊ��acd��a��cd��ef��
(2)��þ�������ã����ձ�����ҺΪϡ���ᣬ�γ�ԭ��ط�Ӧ��þΪ��������Ϊ��������������Mg-2e-=Mg2+���ɹ۲쵽Mg���ܽ⣬AlƬ��������ð����������ָ�뷢��ƫת���ʴ�Ϊ��Mg���ܽ⣬AlƬ��������ð����������ָ�뷢��ƫת��Mg-2e-=Mg2+��
������������������Һ��Ӧ����þ���ܣ����ձ�����ҺΪ����������Һ����Ϊ������þΪ��������������NaAlO2������������������Ӧ���ܷ���ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��Al��2Al+2NaOH+2H2O=2NaAlO2+3H2����

����Ŀ����������ʵ����ʵ�����ܵõ���Ӧ���۵���
ѡ�� | ʵ����������� | ���� |
A | �������Һ�еμ�����Na2SiO3��Һ���۲쵽��ɫ���� | ���������ǿ��H2SiO3 |
B | ���ʢ��2mL5% H2O2��Һ����֧�Թ��зֱ����0.2mol/LFeCl3��0.3mol/LCuCl2��Һ��1mL��ǰ���������ݵ����ʸ��� | ��Ч��: Fe3+> Cu2+ |
C | ��һ��Ũ�ȵĴ�����Һ�м���þ�����������ݵ����ʻ��ȼӿ��ټ��� | ��Ӧ���������ٶȱ仯������Ϊ�������ƽ���������ƶ����������ƶ� |
D | �����·ֱ���Ũ�ȵĴ���Ͱ�ˮpH�����ߵ�pH �ֱ�Ϊ2��12 | �����£�����Ͱ�ˮ�ĵ���ƽ�ⳣ����� |
A. A B. B C. C D. D
����Ŀ����������������ǹ�ҵ����Ҫ�Ļ���ԭ�ϣ�Ҳ��ʵ�����ﳣ���Ļ�ѧ�Լ���
�ⶨ�к��ȣ�
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ__________���к�����ֵΪ57.3kJ/mol����
��2��ȡ50mL 0.5mol/L HCl��Һ��50mL0.55mol/L NaOH��Һ���вⶨ����ʵ����ֵС��57.3kJ/mol��ԭ������_______������ţ���
A�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶�
B����ȡ��������ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D��ʵ��װ�ñ��¡�����Ч����
������к͵ζ���
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000 mol��L-1 HCl����Һ�����к͵ζ�(�÷�̪��ָʾ��)����ش��������⣺
��1����ʽ�ζ���������ˮϴ��������Ӧ�ý��еIJ�����__________________________��
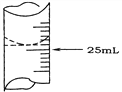
��2������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10 mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ___________��
��ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.11 |
2 | 25.00 | 1.56 | 33.30 |
3 | 25.00 | 0.22 | 26.31 |
��3��ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ________________(С���������λ)��
��4��������Щ������ʹ�ⶨ���ƫ��___________(�����)��
A����ƿ������ˮϴ����ֱ��ע�������Һ���еζ�
B���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ
C����ʽ�ζ���������ˮϴ�Ӻ�����ȡ��25.00mL����Һע����ƿ�н��еζ�
D���ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӵζ��ܶ���
��5���ζ��ﵽ�յ�ı�־��_________________________________________________��
���𰸡� H+(aq)+OH-(aq)= H2O(l)��H=-57.3kJ��mol��1 B �ü�Һ��ϴ 23.80mL 0.1044 mol��L-1 BD �������һ�α�Һ����Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ԭ
�������� I.��1��.ǿ��ǿ����к���Ϊ-57.3kJ/mol����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų�������Ϊ57.3kJ����ϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ��H+(aq)+OH-(aq)= H2O(l) ��H=-57.3kJ��mol��1���ʴ�Ϊ��H+(aq)+OH-(aq)= H2O(l) ��H=-57.3kJ��mol��1��
��2��.A�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶ȣ���������¶ȼ��ϵ�NaOH�������ᷴӦ��ʹ�������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС���к��ȵ���ֵƫС����A��ȷ��B����ȡ��������ʱ���Ӷ������ᵼ������ȡ���������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��B����C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�϶࣬�к��ȵ���ֵƫС����C��ȷ��D��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����D��ȷ����˴�ѡB
II. ��1����ʽ�ζ���������ˮϴ��������Ӧ���ô����NaOH��Һ������ϴ������ὫNaOH��Һϡ�ͣ����ʵ�����ʴ�Ϊ���ü�Һ��ϴ��
��2��.��ͼ��֪���ζ���Һ��Ķ���Ϊ��24.90mL����ζ�ǰ�ζ�����Һ�����Ϊ1.10 mL�����ʱ���ı���Һ�����Ϊ����24.90��1.10��mL=23.80mL���ʴ�Ϊ��23.80mL��
��3��.�ɱ������ݿ�֪����1�����ı�Һ������ǣ���26.11��0.00��mL=26.11mL����2�����ı�Һ������ǣ���33.30��1.56��mL=31.74mL����3�����ı�Һ������ǣ���26.31��0.22��mL=26.09mL�����2���������ϴ���ȥ�������ı�Һ��ƽ�����������26.11+26.09��mL��2=26.10mL������NaOH��Һ�����ʵ���Ũ��Ϊ��c��NaOH��=![]() = 0.1044 mol��L-1���ʴ�Ϊ��0.1044 mol��L-1��
= 0.1044 mol��L-1���ʴ�Ϊ��0.1044 mol��L-1��
��4��. A����ƿ������ˮϴ����ֱ��ע�������Һ���еζ�����ʵ������Ӱ�죬��A����B���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ�������ı�Һ���ƫ�ⶨ���ƫ�ߣ���B��ȷ��C����ʽ�ζ���������ˮϴ�Ӻ�����ȡ��25.00mL����Һע����ƿ�н��еζ�������ȡ����Һƫ�٣����ı�Һ�����ƫ�٣��ⶨ���ƫ�ͣ���C����D���ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӵζ��ܶ�������������ȡ�ı�Һ���ƫ�ⶨ���ƫ�ߣ���D��ȷ����ѡBD��
��5��. ���ζ��յ�ʱ�����������һ�α�Һ����Һ���ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ԭ���ʴ�Ϊ���������һ�α�Һ����Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ԭ��
�����͡�ʵ����
��������
26
����Ŀ����������������Ҫ�Ľ��������ǵĵ��ʼ����������Ÿ��Ե����ʡ�
��1����һ���¶��£�������������һ����̼������Ӧ��Fe2O3(s)��3CO(g) ![]() 2Fe(s)��3CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
2Fe(s)��3CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
�¶�/�� | 1 000 | 1 150 | 1 300 |
ƽ�ⳣ�� | 64.0 | 50.7 | 42.9 |
��ش��������⣺
�ٸ÷�Ӧ��ƽ�ⳣ������ʽK��__________����H_______0(�>������<������)��
������߷�Ӧ��CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________��
A������Fe���� B��������ʵĴ���
C��������������� D�����ͷ�Ӧ���¶�
����һ���ݻ�Ϊ1 L���ܱ������У�1000 ��ʱ����Fe2O3��CO��2 mol����Ӧ����10 min��ﵽƽ�⡣���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ����v(CO)��______________��Fe2O3��ƽ��ת����Ϊ________��
��2������Mg(OH)2��Һ�еμ�FeCl3��Һ,�������ɫ����, ��Ӧ�����ӷ���ʽ��________________________________________��
��3����֪��2Fe(s)+3/2O2(g)=Fe2O3(s) ��H��824 kJ��mol��1��2Al(s)+3/2O2(g)=Al2O3(s) ��H��1675.7 kJ��mol��1��������Fe2O3������Ӧ����Al2O3��Fe���Ȼ�ѧ����ʽΪ ��___________________________��
����Ŀ���ý����ܰ壨������Fe��Ni���Ʊ�Ӧ�ù㷺���Ȼ��ܵĹ����������£�
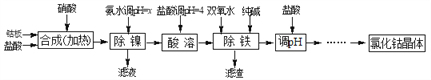
ע���������ᷴӦ������������������ſ��ܽ���ʵ��������
�й��ܡ�����������������ʼ��±���
��ѧʽ | ������ȫʱ��pH | �������� |
Co(OH)2 | 9.4 | Co��2HCl��CoCl2��H2�� Co2����2NH3��H2O��Co(OH)2����2NH4�� Co2����2H2O Ni��2HCl��NiCl2��H2�� Ni2����6NH3��H2O��[Ni(NH3)6]2����6H2O |
Fe(OH)2 | 9.6 | |
Fe (OH)3 | 3.7 |
��1���������������У�NH3��H2O�����Է�Ӧ���ʵ�Ӱ����������ݣ��ӱ������ݿ�֪����PH������x��_______ʱ������Ч����á�
��NH3��H2O��pH | ����/% | Ni2������/% |
9 | 98.1 | 0.08 |
9.5 | 98 | 0.05 |
10 | 97.6 | 0.005 |
10.3 | 94 | 0.005 |
��2����������������������һ����ʱ������ɣ���������н��в���Co(OH)2ת��ΪCo(OH)3���˷�Ӧ�Ļ�ѧ����ʽΪ________________��
��3���������������м���˫��ˮ������Ӧ�����ӷ���ʽ��________________________��
��4���������������м���Ĵ���������________________________________��
��5���ڡ���pH�������У��������������______________________________��
��6����֪25��ʱ��Ksp[Fe(OH)3]��4.0��10��38������¶��·�ӦFe3����3H2O![]() Fe(OH)3��3H����ƽ�ⳣ��Ϊ_____________________��
Fe(OH)3��3H����ƽ�ⳣ��Ϊ_____________________��
����Ŀ�����о����������ȷ��һ����( )
���Ӿ��� | ԭ�Ӿ��� | ���Ӿ��� | |
A | NaOH | Ar | SO2 |
B | H2SO4 | ʯī | S |
C | CH3COONa | SiO2 | CO2 |
D | Ba(OH)2 | ���ʯ | ���� |