题目内容
(16分)有一含NaCl、Na2CO3·10H2O和NaHCO3的混合物,某同学设计如下实验,通过测量反应前后C、D装置质量的变化,测定该混合物中各组分的质量分数。

(1)实验时,B中发生反应的化学方程式为
, 。
(2)装置C、D中盛放的试剂分别为:
C ,D (供选试剂为:浓硫酸、无水CaCl2、碱石灰)
(3)E装置中的仪器名称是 ,它在该实验中的主要作用是
(4)若将A装置换成盛放NaOH溶液的洗气瓶,则测得的NaCl含量将 (填“偏高”、“偏低”或“无影响”)。
(5)若样品的质量为m g,反应后C、D的质量差分别为m1g、m2g,则该混合物中Na2CO3·10H2O的质量分数为: (结果用分数表示,可不化简)。

(1)实验时,B中发生反应的化学方程式为
, 。
(2)装置C、D中盛放的试剂分别为:
C ,D (供选试剂为:浓硫酸、无水CaCl2、碱石灰)
(3)E装置中的仪器名称是 ,它在该实验中的主要作用是
(4)若将A装置换成盛放NaOH溶液的洗气瓶,则测得的NaCl含量将 (填“偏高”、“偏低”或“无影响”)。
(5)若样品的质量为m g,反应后C、D的质量差分别为m1g、m2g,则该混合物中Na2CO3·10H2O的质量分数为: (结果用分数表示,可不化简)。
(1)2NaHCO3  Na2 CO3 + H2O + CO2↑ Na2CO3·10H2O
Na2 CO3 + H2O + CO2↑ Na2CO3·10H2O  Na2CO3 + 10H2O
Na2CO3 + 10H2O
(2)无水CaCl2 碱石灰
(3)(球形)干燥管 防止空气中的CO2和水蒸气进入影响测定结果
(4)偏低 (5)286(m1 -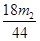 )/180m ×100%
)/180m ×100%
 Na2 CO3 + H2O + CO2↑ Na2CO3·10H2O
Na2 CO3 + H2O + CO2↑ Na2CO3·10H2O  Na2CO3 + 10H2O
Na2CO3 + 10H2O(2)无水CaCl2 碱石灰
(3)(球形)干燥管 防止空气中的CO2和水蒸气进入影响测定结果
(4)偏低 (5)286(m1 -
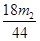 )/180m ×100%
)/180m ×100%试题分析:(1)B中盛放的是固体混合物,所以加热时反应的方程式为2NaHCO3
 Na2 CO3 + H2O + CO2↑、Na2CO3·10H2O
Na2 CO3 + H2O + CO2↑、Na2CO3·10H2O  Na2CO3 + 10H2O。
Na2CO3 + 10H2O。(2)C是吸收水蒸气的,D是吸收CO2的,U形管中不能放液体干燥剂,所以试剂分别是无水CaCl2和碱石灰。
(3)根据E的结构可知,E是干燥管。由于空气中也含有水蒸气和CO2,所以E的作用是防止空气中的CO2和水蒸气进入影响测定结果。
(4)如果是氢氧化钠溶液,则水蒸气的含量将增加,则碳酸钠和碳酸氢钠的质量将增加,所以氯化钠的质量减少。
(5)生成CO2是m2g,则碳酸氢钠产生的水是m2/44×18g,所以碳酸钠生成的水是(m1-m2/44×18)g,所以碳酸钠晶体的质量是286(m1-18m2/44)/180,因此质量分数是286(m1-18m2/44)/180m ×100%。
点评:化学实验解题的关键就是要审清题意,然后分析好各个装置的作用,这样才能轻松解决每个问题。

练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目
 X + CO2 + H2O; ② Z + CO2
X + CO2 + H2O; ② Z + CO2