��Ŀ����
��2010?��Զģ�⣩��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����ã�ij�о���ѧϰС��������ͼװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
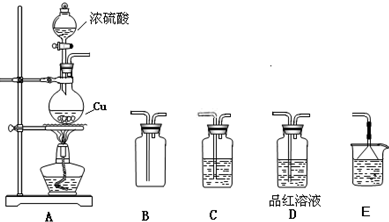
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ
��2����ҪʹB ���ռ��������SO2���壨��֤ʵB�����ռ�������������װ�õ�����˳��Ϊ��
��3��C ��ʢ�ŵ��Լ���
��4������ƿ�г�ַ�Ӧ��ͬѧ�Ƿ���ͭ��ʣ�࣬����ⷢ������Ҳ��ʣ�࣮����������ʣ��ķ�����
��5���ڲ�����Ũ�����ǰ���£�Ϊʹͭ��һ���ܽ⣬������ƿ�м���
������ ��FeSO4 ��Fe2O3 ��KNO3��

��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
��
| ||
��2����ҪʹB ���ռ��������SO2���壨��֤ʵB�����ռ�������������װ�õ�����˳��Ϊ��
A
A
��C
C
��B
B
��D
D
��E
E
������ĸ��ʾ������3��C ��ʢ�ŵ��Լ���
Ũ����
Ũ����
��֤��B �����ռ���SO2��������D��Ʒ����Һ��ɫ
D��Ʒ����Һ��ɫ
����4������ƿ�г�ַ�Ӧ��ͬѧ�Ƿ���ͭ��ʣ�࣬����ⷢ������Ҳ��ʣ�࣮����������ʣ��ķ�����
ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ��
ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ��
����5���ڲ�����Ũ�����ǰ���£�Ϊʹͭ��һ���ܽ⣬������ƿ�м���
�ۢ�
�ۢ�
������ţ��������� ��FeSO4 ��Fe2O3 ��KNO3��
��������1��Cu��Ũ�����ڼ������������ɶ�����������ͭ��ˮ��
��2��ʹB���ռ��������SO2���壬�����ɵĶ����������Ũ����������Ķ����������Ʒ����ȷ��ǰ�漯��ƿ�������壬���ͨ��β������װ�ã�
��3��C��ʢ�ŵ��Լ��Ǹ������֤��B�����ռ���SO2��������Ʒ����ɫ��
��4����Zn��Fe�Ƚϻ��õĽ���������Ĵ��ڣ�
��5���������Խ�ǿ�����ʻ�����������ϡ������һ���γ������Խ�ǿ�����ʣ�
��2��ʹB���ռ��������SO2���壬�����ɵĶ����������Ũ����������Ķ����������Ʒ����ȷ��ǰ�漯��ƿ�������壬���ͨ��β������װ�ã�
��3��C��ʢ�ŵ��Լ��Ǹ������֤��B�����ռ���SO2��������Ʒ����ɫ��
��4����Zn��Fe�Ƚϻ��õĽ���������Ĵ��ڣ�
��5���������Խ�ǿ�����ʻ�����������ϡ������һ���γ������Խ�ǿ�����ʣ�
����⣺��1��Cu��Ũ�����ڼ������������ɶ�����������ͭ��ˮ���䷴Ӧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
��2��ʹB���ռ��������SO2���壬�����ɵĶ����������Ũ����������Ķ����������Ʒ����ȷ��ǰ�漯��ƿ�������壬���ͨ��β������װ�ã�����װ�õ�����˳��Ϊ��A��C��B��D��E���ʴ�Ϊ��A��C��B��D��E��
��3��C��ʢ�ŵ��Լ��Ǹ�������������������������C ��ʢ�ŵ��Լ���Ũ���ᣬ����ƿ�г���������������������Ʒ����Һ��ʹƷ����ɫ������֤��B �����ռ���SO2��������Ʒ����ɫ���ʴ�Ϊ��Ũ���D��Ʒ����Һ��ɫ��
��4����Zn��Fe�Ƚϻ��õĽ���������Ĵ��ڣ�ȡ������Ӧ�����Һ����Zn���������ɣ�˵��������ʣ�࣬�ʴ�Ϊ��ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ�ࣻ
��5���������Խ�ǿ�����ʻ�����������ϡ������һ���γ������Խ�ǿ�����ʣ�����Fe2O3�����ᷴӦ����Fe3+������Cu��Ӧ������KNO3��NO3-��H+����Cu��Ӧ����Ϊʹͭ��һ���ܽ⣬������ƿ�м����Fe2O3 ��KNO3���ʴ�Ϊ���ۢܣ�
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��2��ʹB���ռ��������SO2���壬�����ɵĶ����������Ũ����������Ķ����������Ʒ����ȷ��ǰ�漯��ƿ�������壬���ͨ��β������װ�ã�����װ�õ�����˳��Ϊ��A��C��B��D��E���ʴ�Ϊ��A��C��B��D��E��
��3��C��ʢ�ŵ��Լ��Ǹ�������������������������C ��ʢ�ŵ��Լ���Ũ���ᣬ����ƿ�г���������������������Ʒ����Һ��ʹƷ����ɫ������֤��B �����ռ���SO2��������Ʒ����ɫ���ʴ�Ϊ��Ũ���D��Ʒ����Һ��ɫ��
��4����Zn��Fe�Ƚϻ��õĽ���������Ĵ��ڣ�ȡ������Ӧ�����Һ����Zn���������ɣ�˵��������ʣ�࣬�ʴ�Ϊ��ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ�ࣻ
��5���������Խ�ǿ�����ʻ�����������ϡ������һ���γ������Խ�ǿ�����ʣ�����Fe2O3�����ᷴӦ����Fe3+������Cu��Ӧ������KNO3��NO3-��H+����Cu��Ӧ����Ϊʹͭ��һ���ܽ⣬������ƿ�м����Fe2O3 ��KNO3���ʴ�Ϊ���ۢܣ�
���������⿼����Ũ������Cu�ķ�Ӧʵ�飬�漰��Ӧ����ʽ������ļ��顢����ĸ����ļ���ȣ�֪ʶ��϶࣬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2010?��Զģ�⣩�Ե������Ǵӷ仨��ֲ������ȡ�����������ʣ���ṹ��ͼ��ʾ������������ȷ���ǣ�������
��2010?��Զģ�⣩�Ե������Ǵӷ仨��ֲ������ȡ�����������ʣ���ṹ��ͼ��ʾ������������ȷ���ǣ�������