��Ŀ����
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
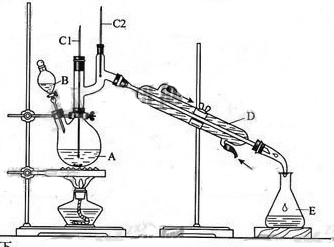
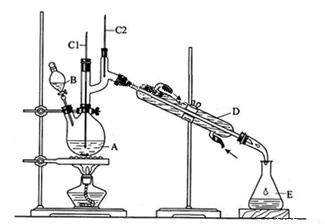
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH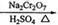 CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
|
|
�е�/�� |
�ܶ�/(g��cm-3) |
ˮ���ܽ��� |
|
������ |
11.72 |
0.8109 |
�� |
|
����ȩ |
75.7 |
0.8017 |
�� |
ʵ�鲽�����£�
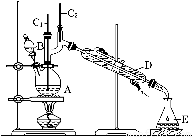
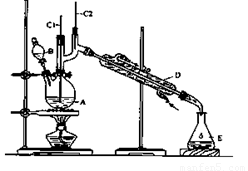
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֡�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4����Һ©��ʹ��ǰ������еIJ����� ������ȷ�𰸱�ţ���
a����ʪ b������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��7����ʵ���У�����ȩ�IJ���Ϊ %��
��1������ �ױŽ���
��2����ֹ���� ��ȴ��
��3����Һ©�� ֱ��������
��4��c
��5����
��6���ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ������
��7��51
��������
��1�����ܽ�Na2Cr2O7��Һ�ӵ�Ũ�����У���ΪŨ������ܶȴ��������Ž���
��2�������ʯ�������Ƿ�ֹ���У������Ⱥ���δ�ӷ�ʯ��Ӧ����ȴ�ӡ�
��3��B�����������Ƿ�Һ©����D����������ֱ�������ܡ�
��4����Һ©��ʹ��ǰ������еĵ�һ������Ǽ�©
��5������ȩ�ܶ�Ϊ0.8017 g��cm-3��С��ˮ���ܶ�
��6��������Ŀ������Ӧ��Ͳ���ķе����ݿ�֪����Ӧ�¶ȱ�����90��95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��7��������ȩ�IJ���Ϊx %�����ݹ�ϵʽ
C4H10O ����C4H8O
74 72
4x 2
��ã�x=51
�����㶨λ���л���ѧʵ�顢��Ӧԭ����������������ѧ����

����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��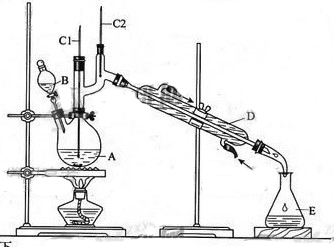
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH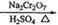 CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| | �е�/�� | �ܶ�/(g��cm-3) | ˮ���ܽ��� |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֡�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4����Һ©��ʹ��ǰ������еIJ����� ������ȷ�𰸱�ţ���
a����ʪ b������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��7����ʵ���У�����ȩ�IJ���Ϊ %��
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH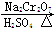 CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/(g��cm��3) | ˮ���ܽ��� |
������ | 117.2 | 0.810 9 | �� |
����ȩ | 75.7 | 0.801 7 | �� |
ʵ�鲽�����£�
��6.0 g Na2 Cr2O7����100 mL�ձ��У���30 mLˮ�ܽ⣬�ٻ�������5 mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0 g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95������E���ռ�90�����µ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0 g��
�ش��������⣺
(1)ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������______________________��
(2)�����ʯ��������________�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������________��
(3)����װ��ͼ�У�B������������________��D������������________��
(4)��Һ©��ʹ��ǰ������еIJ�����________(����ȷ�𰸱��)��
a����ʪ������b���������c����©����d���궨
(5)������ȩ�ֲ�Ʒ���ڷ�Һ©���з���ˮʱ��ˮ��________��(����������������)��
(6)��Ӧ�¶�Ӧ������90��95������ԭ����__________________________________��
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
�����ķ�Ӧ���£�
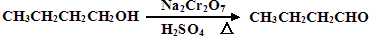
��Ӧ��Ͳ������������б����£�
|
|
�е�/�� |
�ܶ�/g��cm-3 |
ˮ���ܽ��� |
|
������ |
117.2 |
0.8109 |
�� |
|
����ȩ |
75.7 |
0.8017 |
�� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g���ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�����ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡�����
��5����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��6����ʵ���У�����ȩ�IJ���Ϊ ����
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH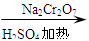 CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
|
|
�е�/��c |
�ܶ�/(g��cm-3) |
ˮ���ܽ��� |
|
������ |
11.72 |
0.8109 |
�� |
|
����ȩ |
75.7 |
0.8017 |
�� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У���˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4����Һ©��ʹ��ǰ������еIJ����� ������ȷ�𰸱�ţ���
a.��ʪ b.���� c.��© d.�궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��7����ʵ���У�����ȩ�IJ���Ϊ %��