��Ŀ����
��22�֣�
(��)�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6��.
�ű�������ɵñ�ϩ��
��֪��C3H8(g) CH4(g)��HC
CH4(g)��HC CH(g)��H2(g)
CH(g)��H2(g)
 kJ/mol
kJ/mol
CH3CH CH2(g)
CH2(g) CH4(g)��HC
CH4(g)��HC CH(g)
CH(g)  kJ/mol
kJ/mol
����ͬ�����£���ӦC3H8(g) CH3CH
CH3CH CH2(g)��H2(g)��
CH2(g)��H2(g)�� kJ/mol��
kJ/mol��
���Ա���Ϊȼ����������ȼ�ϵ�أ���ص�һ��ͨ��O2��CO2����һ��ͨ����飬�����������̼���Ρ�ͨ�����һ���ǵ�ص� ������ظ����ĵ缫��ӦʽΪ ���ŵ�ʱ��CO32�������ص� �����������������
(��)����ͼװ����ʾ��C��D��E��F���Ƕ��Ե缫���ס�������Һ����������ʵ���Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ��

��A���ǵ�Դ�� ����
�����ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ ��
�����ñ�װ�ø�ͭ����������HӦ���� ����Ʋ�������Ƽ����������Һ�� ��Һ��
��������Һ��c(OH��)=0.1mol/L����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ ��������Һ��c(H+) ����������С�����䡱����
����ͼ��װ�õ��CuSO4��Һһ��ʱ�����������Һ�м���0.2mol Cu(OH)2��
ǡ��ʹ��Һ�ָ������ǰ��Ũ�ȡ�����װ���У���������Cl2���ܽ⼰���ķ�Ӧ����װ�ù��������� L����״������
��22�֣�
����1����+124.2
��2������ C3H8��10CO32����20e��===4H2O��13CO2 ��
����1����������
��2����1��2��2��2
��3�����Ƽ� AgNO3
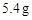 ���
���
��4����14.65
��������

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�