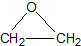
��Ŀ����
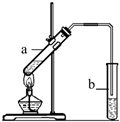
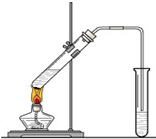 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺��1���Ҵ�����������еĹ����ŷֱ���
�ǻ�
�ǻ�
���Ȼ�
�Ȼ�
��2��װ���е���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ
����
����
����3������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����
��ֹ����
��ֹ����
����4���÷�Ӧ�������෴Ӧ����
BDE
BDE
��A���ӳɷ�Ӧ B��ȡ����Ӧ C��ˮ�ⷴӦ D��������Ӧ E�����淴Ӧ
��5��д����ȡ���������Ļ�ѧ��Ӧ����ʽ
CH3COOH+CH3CH2OH
CH3COOC2H5+H2O
| Ũ���� |
| �� |
CH3COOH+CH3CH2OH
CH3COOC2H5+H2O
��| Ũ���� |
| �� |
��6����ˮ�����ֳƱ����ᣨ�۵�16.6�棩�������½ϵ�ʱ����ˮ����ͻ���������һ���ľ��壮���˵����ʵ�����������������ʱ���㽫��δ��Լ�ƿ��ȡ����ˮ���
ˮԡ�ȡ�����ë���ȷ�
ˮԡ�ȡ�����ë���ȷ�
����7���Ҵ���һ�������»��ܷ�����ˮ��Ӧ������ϩ����ϩͨ��������Ȼ�̼��Һ�У��۲쵽��������
��ˮ��ɫ��ȥ
��ˮ��ɫ��ȥ
���䷴Ӧ����ʽΪCH2=CH2+Br-Br��CH2Br-CH2Br
CH2=CH2+Br-Br��CH2Br-CH2Br
�����⣬��ϩ�����������������飬����������Ҫ�����֣�
����һ��
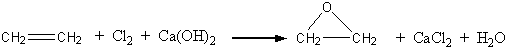
���ն���
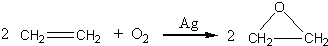
������ɫ��ѧ��ԭ�����������������ԭ�Ӿ����Ժõķ�Ӧ����ˣ���ʵ�������У�Ӧ����
���ն�
���ն�
�������һ�����ն������������������ã���������1�����Ĺ�����Ϊ�ǻ�������Ĺ��������Ȼ���
��2�������Թ����Ȳ����������Թ��еĵ�������Һ���¿��ܷ���������
��3��Һ�����Ҫ�����Ƭ����ֹ���У�
��4��������Ҵ���Ũ���������·���������Ӧ����������������ˮ������������Ӧ��
��5��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��6����������۵�ͣ�
��7����ϩ����ˮ�����ӳɷ�Ӧ����ɫ��ȥ��
�����ԭ�Ӿ����Է�Ӧ��ԭ�Ϸ����е�ԭ��ȫ��ת��������Ҫ�IJ�������������ʵ�����ŷţ�
��2�������Թ����Ȳ����������Թ��еĵ�������Һ���¿��ܷ���������
��3��Һ�����Ҫ�����Ƭ����ֹ���У�
��4��������Ҵ���Ũ���������·���������Ӧ����������������ˮ������������Ӧ��
��5��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��6����������۵�ͣ�
��7����ϩ����ˮ�����ӳɷ�Ӧ����ɫ��ȥ��
�����ԭ�Ӿ����Է�Ӧ��ԭ�Ϸ����е�ԭ��ȫ��ת��������Ҫ�IJ�������������ʵ�����ŷţ�
����⣺��1�����Ĺ�����Ϊ�ǻ����Ҵ��к��еĹ��������ǻ�������Ĺ��������Ȼ����������к��еĹ��������Ȼ���
�ʴ�Ϊ���ǻ����Ȼ���
��2�����������Ʊ��Թ����Ȳ�������������Һ���¿��ܷ����������ʵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ������
�ʴ�Ϊ����ֹ������
��3��Һ�����Ҫ�����Ƭ����ֹ���У�
�ʴ�Ϊ����ֹ���У�
��4��������Ҵ���Ũ���������·���������Ӧ����������������ˮ����Ӧ����ʽΪCH3COOH+CH3CH2OH
CH3COOC2H5+H2O�������е��ǻ���-OC2H5ȡ��������ȡ����Ӧ��ͬʱ�÷�ӦΪ���淴Ӧ��
��ѡ��BDE��
��5��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��Ӧ����ʽΪCH3COOH+CH3CH2OH
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O��
��6����������۵�16.6�棬���½ϵ�ʱ����ˮ����ͻ���������һ���ľ��壮���Կ��Ѹ���������Լ�ƿ��ˮԡ�����Ȼ�������ë�����ȵ�ʹ���ڻ���
����ȷ�𰸣�ˮԡ�ȡ�����ë���ȷ�ȣ�
��7����ϩ����ˮ�����ӳɷ�Ӧ������1��2-�������飬��ˮ��ɫ��ȥ����Ӧ����ʽΪCH2=CH2+Br-Br��CH2Br-CH2Br��
����һ�������ɻ����������CaCl2��H2O�����ɣ���ʹ���ж�������������������ɫ��ѧ��ԭ��
���ն�����ϩ��������Ӧȫ�������˻������飬ԭ��������Ϊ100%��������ɫ��ѧ��ԭ��
�ʴ�Ϊ����ˮ��ɫ��ȥ��CH2=CH2+Br-Br��CH2Br-CH2Br�����ն���
�ʴ�Ϊ���ǻ����Ȼ���
��2�����������Ʊ��Թ����Ȳ�������������Һ���¿��ܷ����������ʵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ������
�ʴ�Ϊ����ֹ������
��3��Һ�����Ҫ�����Ƭ����ֹ���У�
�ʴ�Ϊ����ֹ���У�
��4��������Ҵ���Ũ���������·���������Ӧ����������������ˮ����Ӧ����ʽΪCH3COOH+CH3CH2OH
| Ũ���� |
| �� |
��ѡ��BDE��
��5��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��Ӧ����ʽΪCH3COOH+CH3CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
��6����������۵�16.6�棬���½ϵ�ʱ����ˮ����ͻ���������һ���ľ��壮���Կ��Ѹ���������Լ�ƿ��ˮԡ�����Ȼ�������ë�����ȵ�ʹ���ڻ���
����ȷ�𰸣�ˮԡ�ȡ�����ë���ȷ�ȣ�
��7����ϩ����ˮ�����ӳɷ�Ӧ������1��2-�������飬��ˮ��ɫ��ȥ����Ӧ����ʽΪCH2=CH2+Br-Br��CH2Br-CH2Br��
����һ�������ɻ����������CaCl2��H2O�����ɣ���ʹ���ж�������������������ɫ��ѧ��ԭ��
���ն�����ϩ��������Ӧȫ�������˻������飬ԭ��������Ϊ100%��������ɫ��ѧ��ԭ��
�ʴ�Ϊ����ˮ��ɫ��ȥ��CH2=CH2+Br-Br��CH2Br-CH2Br�����ն���
���������⿼�������������Ʊ���ע��ʵ����Һ�����ơ�������Ӧ�Ļ�����

��ϰ��ϵ�д�
�����Ŀ
 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺ CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺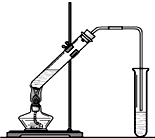 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺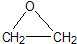 +CaCl2+H2O
+CaCl2+H2O