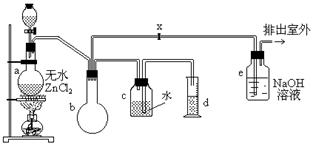
��Ŀ����
һ�ȼ��飬Ҳ�м��ȣ�����������ɫ���壬�ܶ�Ϊ0.9159��/����3���۵�Ϊ��97.73�棬�е�Ϊ��24.2�棬18��ʱ��ˮ�е��ܽ��Ϊ280����/����ˮ�������ѡ���ͪ���ܣ��������Ҵ�����ʵ�����������ͼװ����ȡһ�ȼ��飺

�Ʊ�װ�õķ�Һ©������ƿ�зֱ�ʢ�м״���Ũ���ᡣ
����д���пհף�
��1���Ʊ�һ�ȼ���Ļ�ѧ����ʽ ��
��2��װ��b�������� ��
��3����֪±�����ܷ�������ˮ�⣬ת��Ϊ����װ��e�п��ܷ����ķ�Ӧ����ʽΪ
��
��4�������������CH3Cl�ķ����ǣ���e���ݳ��ڵ�ȼCH3Cl���壬���������ɫ����������ȼ���������������𣩡�CH3Clȼ�յĻ�ѧ����ʽ�� ��
��5��ʵ����ʵ�ʰ��״���Ũ����1�U2��������֮�ȣ����з�Ӧ�������� ��
��6��ijѧ���ڹرջ���x�����������ʵ��ʱ���֣��ռ���һ�������������������ĵļ״���Ũ����Ļ��Һ��������������¶ࣨװ�õ�������û�����⣩����ԭ���� ��
��7��ʵ�������d���ռ�����Һ���к��� ��
��1��CH3OH��HCl CH3Cl��H2O
CH3Cl��H2O
��2����ֹ��������֤��ȫ ��3��NaOH��HCl��NaCl��H2O
��4��2CH3Cl��3O2 2CO2��2H2O��2HCl
2CO2��2H2O��2HCl
��5����ʹ�״���ַ�Ӧ��ת����һ�ȼ���
��6���״��е�ϵͣ�64.7�棩������ʱ�ӷ�����ʹһ����δ��Ӧ���ݳ�
��7���״������ᡢһ�ȼ���
��������
�����������1���ڴ����������£��״����Ȼ��ⷢ��ȡ����Ӧ����һ�ȼ��飬��Ӧ�Ļ�ѧ����ʽʹCH3OH��HCl CH3Cl��H2O��
CH3Cl��H2O��
��2�������Ȼ��⼫�ӷ������Ȼ����ּ����ܽ���ˮ�У�����װ��b�������Ƿ�ֹ��������֤��ȫ��
��3��±�����ܷ�������ˮ�⣬ת��Ϊ�������������ɴ���ͬʱ���������Ȼ��⣬��װ��e�п��ܷ����ķ�Ӧ����ʽΪNaOH��HCl��NaCl��H2O��
��4������һ�ȼ�������Ԫ�ؿ�֪��һ�ȼ���ȼ�յ�������Ӧ����CO2��ˮ���Ȼ��⣬��CH3Clȼ�յĻ�ѧ����ʽ��2CH3Cl��3O2 2CO2��2H2O��2HCl��
2CO2��2H2O��2HCl��
��5��ʵ����ʵ�ʰ��״���Ũ����1�U2��������֮�ȣ����з�Ӧ����Ȼ�����ǹ����ģ���˿�����״���ת���ʣ�ʹ�״���ַ�Ӧ��ת����һ�ȼ��顣
��6��������֪������֪���״��е�ϵͣ�64.7�棩������ʱ�ӷ�����ʹһ����δ��Ӧ���ݳ��������ռ���һ�������������������ĵļ״���Ũ����Ļ��Һ��������������¶ࡣ
��7�����ڼ״������ᶼ���ӷ��ģ����ʵ�������d���ռ�����Һ���к��м״������ᡢһ�ȼ��顣
���㣺���������Ʊ����й�ʵ����ơ������������Լ��йط�Ӧʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��ʵ�����������������ѧ����ѧ������������������ѧ���淶���Ͻ���ʵ����ơ����������Լ���������������������Ҫ��ȷ���������͵�������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

 53���ò�ϵ�д�
53���ò�ϵ�д� ���۵�Ϊ
���۵�Ϊ �棬�е�Ϊ
�棬�е�Ϊ �棬
�棬 ��ʱ��ˮ�е��ܽ��Ϊ280mL/L(ˮ)�������ѡ���ͪ���ܣ��������Ҵ���
��ʱ��ˮ�е��ܽ��Ϊ280mL/L(ˮ)�������ѡ���ͪ���ܣ��������Ҵ���
 ���������Իش�
���������Իش�