��Ŀ����
(1)�ƣ�Y���Ǽ�����Ϻͳ��������е���ҪԪ�ء����ƿ�ʯ�ɷ���Be2YxFeSi2O10���ÿ�ʯ������ռ�ۺ�ˮ�ܽ⣬���ó�������Y(OH)3��Fe2O3����֪�ڿ������ۻ�ʱ��ʯ��ֻ��Fe�ɣ�2�۱�ɣ�3�ۣ�����x����_______�����������������ʽ��ʾ����ɣ���ѧʽΪ_______________��
��2����SO2ͨ��Fe(NO3)3��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ���������ֱ�Ϊ�ػ�ɫ����ʱ������BaCl2��Һ����������ɫ�������������仯������
��Һ���ػ�ɫ��Ϊdz��ɫ�����ӷ���ʽ��ʾΪ______________________________��������dz��ɫ��Ϊ�ػ�ɫ�����ӷ���ʽΪ______________________________��
��2����SO2ͨ��Fe(NO3)3��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ���������ֱ�Ϊ�ػ�ɫ����ʱ������BaCl2��Һ����������ɫ�������������仯������
��Һ���ػ�ɫ��Ϊdz��ɫ�����ӷ���ʽ��ʾΪ______________________________��������dz��ɫ��Ϊ�ػ�ɫ�����ӷ���ʽΪ______________________________��
��1��2��2BeOFeOY2O32SiO2
��2��SO2+2Fe3++2H2O=SO42-+2Fe2++4H+��3Fe2++NO3-+4H+=3Fe3++NO+2H2O
��2��SO2+2Fe3++2H2O=SO42-+2Fe2++4H+��3Fe2++NO3-+4H+=3Fe3++NO+2H2O
��

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
 H2S +2OH��
H2S +2OH�� 2Cu+4H++O2��
2Cu+4H++O2��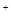 =HCO3-
=HCO3-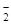 + H2��
+ H2�� 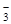
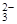 ��2H2O
��2H2O