��Ŀ����
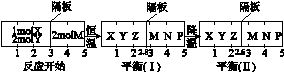
��һ�ܱ������г���1mol H2��1mol I2��ѹǿΪp��Pa��������һ���¶���ʹ�䷢����Ӧ��H2��g��+I2��g��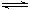 2HI��g������H��0����Ӧ�ﵽƽ��ı�������������ʹ��Ӧ���ʱ������ǣ�N2���μӷ�Ӧ��
2HI��g������H��0����Ӧ�ﵽƽ��ı�������������ʹ��Ӧ���ʱ������ǣ�N2���μӷ�Ӧ��
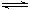 2HI��g������H��0����Ӧ�ﵽƽ��ı�������������ʹ��Ӧ���ʱ������ǣ�N2���μӷ�Ӧ��
2HI��g������H��0����Ӧ�ﵽƽ��ı�������������ʹ��Ӧ���ʱ������ǣ�N2���μӷ�Ӧ��| A�����������ݻ����䣬�����м���1mol H2 |
| B�����������ݻ����䣬�����м���1mol N2 |
| C������������ѹǿ���䣬�����м���1mol N2 |
| D������������ѹǿ���䣬�������ټ���1mol H2��1mol I2 |
C
��

��ϰ��ϵ�д�
�����Ŀ
 xC(g)+2D(g)������Ӧ�ﵽƽ��ʱ������0.4 mol D�������C��ƽ��Ũ��Ϊ0.4 mol/L�����������в���ȷ����
xC(g)+2D(g)������Ӧ�ﵽƽ��ʱ������0.4 mol D�������C��ƽ��Ũ��Ϊ0.4 mol/L�����������в���ȷ���� CO2��H2���ﵽƽ��ʱ����0.7mol��CO2��������������CO����ʼ�����䣬һ��ʼ�ͼ���4mol H2O(g)����ﵽƽ��ʱ���ɵ�CO2������
CO2��H2���ﵽƽ��ʱ����0.7mol��CO2��������������CO����ʼ�����䣬һ��ʼ�ͼ���4mol H2O(g)����ﵽƽ��ʱ���ɵ�CO2������ ��Y��Z��ʾԪ�ط��ţ�����������ʵ�����������Ũ�ȵĴ�С˳��Ӧ���ʵĻ�ѧʽ����Һ��pH������д���пհף�Ҫ����д�й����ӻ�ѧʽ����
��Y��Z��ʾԪ�ط��ţ�����������ʵ�����������Ũ�ȵĴ�С˳��Ӧ���ʵĻ�ѧʽ����Һ��pH������д���пհף�Ҫ����д�й����ӻ�ѧʽ���� Z(g)+2W(g) ��H��0���ﵽƽ�⣨��ʱ��B���ܶȱ�Ϊԭ����
Z(g)+2W(g) ��H��0���ﵽƽ�⣨��ʱ��B���ܶȱ�Ϊԭ���� ���Իش�
���Իش�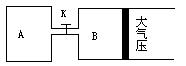
 ��H2(g)��CO2(g)
��H2(g)��CO2(g) H2O(g)��CO(g)��ƽ�ⳣ��K��
H2O(g)��CO(g)��ƽ�ⳣ��K�� �����¶����ڼס��ҡ������������ܱ������У�Ͷ��H2(g)��CO2(g)������ʼŨ�����±���ʾ��
�����¶����ڼס��ҡ������������ܱ������У�Ͷ��H2(g)��CO2(g)������ʼŨ�����±���ʾ�� 0
0 2Z(g) ����2M(g)
2Z(g) ����2M(g)