��Ŀ����
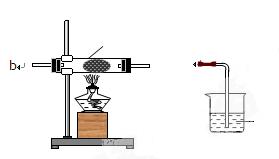
(12��)ij��ѧ��ȤС���ͬѧ����ͼ��ʾʵ��װ�ý���ʵ���о�(ͼ��a��b��c��ʾֹˮ��)������䷽���������ƻ�����;
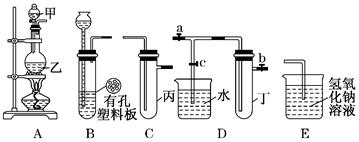
(1)ʵ���ҽ�B��C��E�������� �� ����д���ƣ�Ϊԭ�Ͽ���ȡCl2��Ϊ�������о������Ļ�ѧ����������
(2) ����ʵ���ҳ��÷�����ȡ��������A��C��E�������ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ����������������£�
��������:ʵ�����Ƴ���Ӧ�����Ƿ������ ________________��������������˵�����ɣ�����������������д���У���___________________________________________.
ʵ����Ƴ���Ӧ�Ľ����Ƿ������ ________________������������˵�����ɣ���������������д���У���____________________________________________________.
(3)A��C��E�����������һ����ʵ�飬����֤Cl����Br���Ļ�ԭ��ǿ�����йط�Ӧ�����ӷ���ʽΪ��____________________________________��
(4)B��D��Eװ����������B��ʢװŨ�����ͭƬ(�����п����ϰ���)�����Ƶò�����NO2�й�ʵ�飮
��B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��________���ٴ�ֹˮ��________��ʹ�ձ��е�ˮ�����Թܶ��Ŀ��ܵIJ����� ��

(1)ʵ���ҽ�B��C��E�������� �� ����д���ƣ�Ϊԭ�Ͽ���ȡCl2��Ϊ�������о������Ļ�ѧ����������
(2) ����ʵ���ҳ��÷�����ȡ��������A��C��E�������ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ����������������£�
| ʵ�� ��� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | Ʒ����Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ�� �ݲ��� | ������ˮ��Ӧ�IJ���������� |
ʵ����Ƴ���Ӧ�Ľ����Ƿ������ ________________������������˵�����ɣ���������������д���У���____________________________________________________.
(3)A��C��E�����������һ����ʵ�飬����֤Cl����Br���Ļ�ԭ��ǿ�����йط�Ӧ�����ӷ���ʽΪ��____________________________________��
(4)B��D��Eװ����������B��ʢװŨ�����ͭƬ(�����п����ϰ���)�����Ƶò�����NO2�й�ʵ�飮
��B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��________���ٴ�ֹˮ��________��ʹ�ձ��е�ˮ�����Թܶ��Ŀ��ܵIJ����� ��
(12��) 26.(1)������ơ�Ũ���ᣨ�����������Ĵ𰸣���2�֣�(2) ��������û������֤�������������Ư���ԡ�
����������ȡ�������к���HCl���壬HCl����ˮ������̼�����Ʒ�ĩ��Ӧ�������ݣ�4�֣�
(3)MnO2 +4H+ +2Cl- Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�
Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�
(4)��Cu��4HNO3(Ũ) �� Cu(NO3)2��2NO2����2H2O��2�֣�
��a��b����c��˫�ֽ���(����)�Թܶ���ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ��У�2�֣��������������Ĵ𰸣�
����������ȡ�������к���HCl���壬HCl����ˮ������̼�����Ʒ�ĩ��Ӧ�������ݣ�4�֣�
(3)MnO2 +4H+ +2Cl-
 Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�
Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�(4)��Cu��4HNO3(Ũ) �� Cu(NO3)2��2NO2����2H2O��2�֣�
��a��b����c��˫�ֽ���(����)�Թܶ���ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ��У�2�֣��������������Ĵ𰸣�
��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
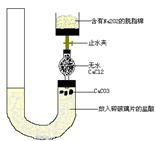
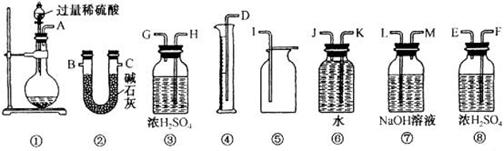
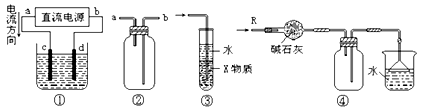
 ������˳�� ��
������˳�� ��  ��
��
 2CrO42-+2H+
2CrO42-+2H+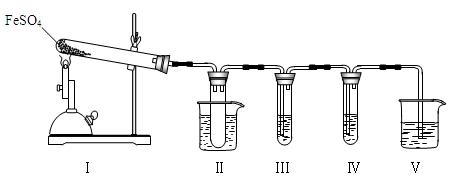
 �����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
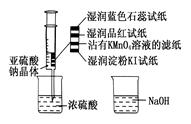

 ��ʵ���й۲�
��ʵ���й۲� ��E���к���ɫ������֣�֤����������_________�ԣ�
��E���к���ɫ������֣�֤����������_________�ԣ�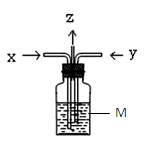 ��
�� 