��Ŀ����
��A��B����������ɵĻ�����壬��ƽ����Է���������A�����ʵ��������仯��ϵ��ͼ����ʾ��
��1��A����Է�������Ϊ___________��
��2������û�������н���������Ԫ�أ�A��B��������ʱ����ѧʽ�ֱ�Ϊ___________��___________��A��B�����л���ʱ����ѧʽ�ֱ�Ϊ___________��___________��
��3��ij�������A��B�������϶��ɣ�����������������ϣ���ȼ��Ӧ����ʣ�࣬����������ͨ������ŨH2SO4�����ٵ����ǡ�õ��������������������������ʯ�ң�������룩����֪ͨ��ŨH2SO4��ͨ����ʯ�������ٵ��������֮��Ϊ5��2������������ڱ�״���²ⶨ���ݴ�ȷ������������ɡ�
��1��44 ��2��N2O NO C3H8 C2H6 ��3��C3H8��CH2O��C2H6��C2H4O
��������1������ƽ����Է��������ĺ���ã�
 ���
���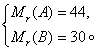
(2)A��B��������Ԫ����ɣ���Mr��A��=44��Mr��B��=30�����A��B��Ϊ����ʱ��AΪN2O��BΪNO��A��B��Ϊ�л���ʱ��AΪC3H8��BΪC2H6��
��3����Ϊ���������A��B�������ϣ���˻�������ƽ����Է�������Ϊ![]() =37������Ŀ����֪���������ȼ�ղ�����n(H2O)��n(CO2)=5��2,��n��CO2��=n(CO)��
=37������Ŀ����֪���������ȼ�ղ�����n(H2O)��n(CO2)=5��2,��n��CO2��=n(CO)��
���������ƽ�����ΪCxHyOz,��ȼ�յĻ�ѧ����ʽΪ��
CxHyOz+![]() O2
O2![]()
![]() CO2+
CO2+![]() CO+
CO+![]() H2O
H2O
��� ���
���
���Ի�������ƽ�����ΪC2H5O0.5��
����ƽ����ɵĺ��弰Mr(A)=44,Mr(B)=30,�Ƶû���������ΪC3H8��CH2O��C2H6��C2H4O��

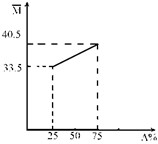 ��A��B����������ɵĻ�����壬��ƽ����Է���������A�����ʵ��������仯��ϵ��ͼ��ʾ��
��A��B����������ɵĻ�����壬��ƽ����Է���������A�����ʵ��������仯��ϵ��ͼ��ʾ��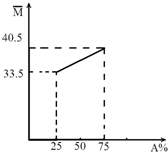 ��A��B����������ɵĻ�����壬��ƽ����Է���������A�����ʵ��������仯��ϵ��ͼ��ʾ��
��A��B����������ɵĻ�����壬��ƽ����Է���������A�����ʵ��������仯��ϵ��ͼ��ʾ��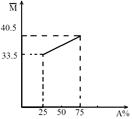
 ����ϣ���ȼ��Ӧ�����ʣ�࣬����������ͨ������ŨH2SO4�����ٵ����ǡ�õ��������������������������ʯ��(�������)����֪ͨ��ŨH2SO4��ͨ����ʯ�������ٵ��������֮��Ϊ5��2�������������105���1.01��105Pa�����²ⶨ�ģ��ݴ�ȷ�������������Ϊ ____________________________________________________________ ��
����ϣ���ȼ��Ӧ�����ʣ�࣬����������ͨ������ŨH2SO4�����ٵ����ǡ�õ��������������������������ʯ��(�������)����֪ͨ��ŨH2SO4��ͨ����ʯ�������ٵ��������֮��Ϊ5��2�������������105���1.01��105Pa�����²ⶨ�ģ��ݴ�ȷ�������������Ϊ ____________________________________________________________ ��