��Ŀ����
����ѡһ�����л���ѧ������
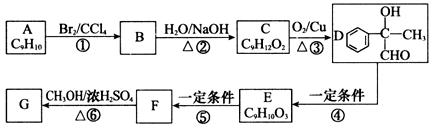
��ʯ���ѽ����A�ɺϳɶ��ֲ����F��һ�ֳ�Ϊ�������������ɱ����������ͼ��A�ϳ�һЩ�л������ת����ϵ��
��ʯ���ѽ����A�ɺϳɶ��ֲ����F��һ�ֳ�Ϊ�������������ɱ����������ͼ��A�ϳ�һЩ�л������ת����ϵ��

��1��������Ӧ�����ڼӳɷ�Ӧ����___________��
��2��1H�˴Ź�����ͼ����A����ֻ�����ֻ�ѧ������ͬ����ԭ�ӣ���������о�����A�����к�������̼̼˫����д��A��D�Ľṹ��ʽ��A__________��D_____________
��3��д��Bת��ΪC�Ļ�ѧ����ʽ__________________________________________��
��4��H������Ũ��������¿ɷֱ�����һ�ֺ���Ԫ������Ԫ������Ԫ���IJ��д��������Բ��������ط�Ӧ�Ļ�ѧ����ʽ______________________________________��
��5��д����A��Ϊͬ���칹�壬�ҷ�������4��̼ԭ�ӹ�ֱ�ߵ��л���Ľṹ��ʽ________________________________��
��6��D��������HOOCCH2CHClCOOH�Ĺ����л����м�������ɣ��м����Ľṹ��ʽ������_________________��д��һ�֣�����������ʴ��ڵ��Լ���________________��
��2��1H�˴Ź�����ͼ����A����ֻ�����ֻ�ѧ������ͬ����ԭ�ӣ���������о�����A�����к�������̼̼˫����д��A��D�Ľṹ��ʽ��A__________��D_____________
��3��д��Bת��ΪC�Ļ�ѧ����ʽ__________________________________________��
��4��H������Ũ��������¿ɷֱ�����һ�ֺ���Ԫ������Ԫ������Ԫ���IJ��д��������Բ��������ط�Ӧ�Ļ�ѧ����ʽ______________________________________��
��5��д����A��Ϊͬ���칹�壬�ҷ�������4��̼ԭ�ӹ�ֱ�ߵ��л���Ľṹ��ʽ________________________________��
��6��D��������HOOCCH2CHClCOOH�Ĺ����л����м�������ɣ��м����Ľṹ��ʽ������_________________��д��һ�֣�����������ʴ��ڵ��Լ���________________��
��1���٢�
��2��CH2=CHCH=CH2��HOCH2CH2CHClCH2OH
��3��ClCH2CH=CHCH2Cl+2NaOH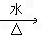 HOCH2CH=CHCH2OH+2NaCl
HOCH2CH=CHCH2OH+2NaCl
��4��2HOOCCH2CH(OH)COOH
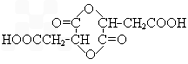 +2H2O
+2H2O
��5��CH3C��CCH3
��6��OHCCH2CHClCH2OH��HOCH2CH2CHClCHO��OHCCH2CHClCHO�� HOOCCH2CHClCHO��OHCCH2CHClCOOH����дһ�֣�������������ͭ����Һ����������Һ
��2��CH2=CHCH=CH2��HOCH2CH2CHClCH2OH
��3��ClCH2CH=CHCH2Cl+2NaOH
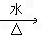 HOCH2CH=CHCH2OH+2NaCl
HOCH2CH=CHCH2OH+2NaCl��4��2HOOCCH2CH(OH)COOH

 +2H2O
+2H2O��5��CH3C��CCH3
��6��OHCCH2CHClCH2OH��HOCH2CH2CHClCHO��OHCCH2CHClCHO�� HOOCCH2CHClCHO��OHCCH2CHClCOOH����дһ�֣�������������ͭ����Һ����������Һ

��ϰ��ϵ�д�
�����Ŀ


 ��CH2��CHCOO
��CH2��CHCOO
 ������ͬ����ͬ��������
������ͬ����ͬ��������
