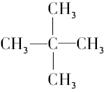
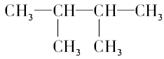��Ŀ����
̼���⡢��3��Ԫ����ɵ��л���A����Է�������Ϊ102���������������Ϊ9.8%����������ԭ�Ӹ���Ϊ����5����
(1��A�ķ���ʽ��______________��
(2��A��2����ͬ�ĺ��������ţ���������___________��
(3��һ�������£�A��������Ӧ����B��B���ӵĽṹ����Ϊ1��̼ԭ��������2����������2���ṹ��ͬ�Ļ��š�
��A�Ľṹ��ʽ��___________��
��A���ܷ����ķ�Ӧ�ǣ���д�����ĸ��___________��
a.ȡ����Ӧ b.��ȥ��Ӧ
c.������Ӧ d.��ԭ��Ӧ
(4��д��������A������ͬ�����Ų�����֧����ͬ���칹��Ľṹ��ʽ___________��___________��
(5��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ��������һ�ֵķ�������2�������˷�Ӧ�Ļ�ѧ����ʽ��__________________��
��6����֪�����ȱ�������Ҷ����������¾ۺϷ�Ӧ��
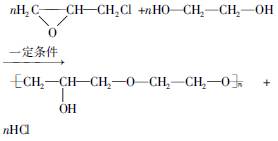
BҲ���뻷���ȱ��鷢�����Ʒ�Ӧ���ɸ߾���ø߾���Ľṹ��ʽ��______________��
(1��A�ķ���ʽ��______________��
(2��A��2����ͬ�ĺ��������ţ���������___________��
(3��һ�������£�A��������Ӧ����B��B���ӵĽṹ����Ϊ1��̼ԭ��������2����������2���ṹ��ͬ�Ļ��š�
��A�Ľṹ��ʽ��___________��
��A���ܷ����ķ�Ӧ�ǣ���д�����ĸ��___________��
a.ȡ����Ӧ b.��ȥ��Ӧ
c.������Ӧ d.��ԭ��Ӧ
(4��д��������A������ͬ�����Ų�����֧����ͬ���칹��Ľṹ��ʽ___________��___________��
(5��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ��������һ�ֵķ�������2�������˷�Ӧ�Ļ�ѧ����ʽ��__________________��
��6����֪�����ȱ�������Ҷ����������¾ۺϷ�Ӧ��
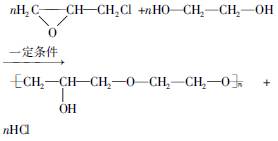
BҲ���뻷���ȱ��鷢�����Ʒ�Ӧ���ɸ߾���ø߾���Ľṹ��ʽ��______________��
(1)C5H10O2 (2)�ǻ���ȩ��
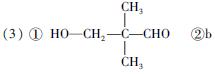
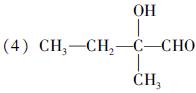
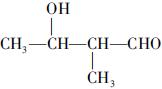
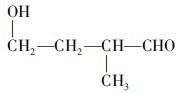
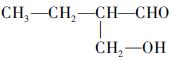
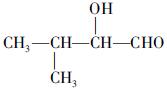
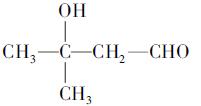
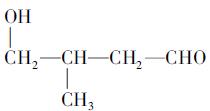
(�����������2������)
(5)CH3COOCH(CH3)2+H2O CH3COOH+(CH3)2CH��OH
CH3COOH+(CH3)2CH��OH
(6)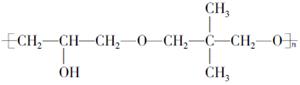

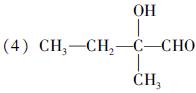

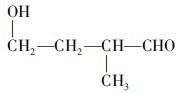
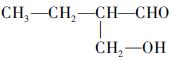


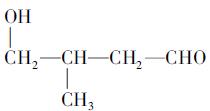
(�����������2������)
(5)CH3COOCH(CH3)2+H2O
 CH3COOH+(CH3)2CH��OH
CH3COOH+(CH3)2CH��OH(6)
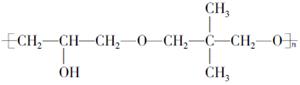
��1���������֪һ����A�к�Hԭ�Ӹ���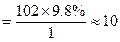 ����Oԭ�Ӹ���Ϊ2��Cԭ�Ӹ���
����Oԭ�Ӹ���Ϊ2��Cԭ�Ӹ���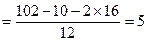 ������A�ķ���ʽΪC5H10O2��2����ͬ�ĺ�����������ȩ�������ǻ�����3��A��������Ӧ����B��֪��B�ķ���ʽΪC5H12O2�������������ǻ�������B�ĽṹΪ
������A�ķ���ʽΪC5H10O2��2����ͬ�ĺ�����������ȩ�������ǻ�����3��A��������Ӧ����B��֪��B�ķ���ʽΪC5H12O2�������������ǻ�������B�ĽṹΪ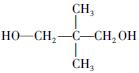 ����A�ĽṹΪ
����A�ĽṹΪ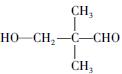 ,��Ϊ�봼�ǻ����ڵ�̼ԭ��������ԭ�ӣ����Բ��ܷ�����ȥ��Ӧ����4������ʽΪC5H10O2����������3��̼ԭ�ӵ��л���ΪA��������A������ͬ�����Ų�����֧����ͬ���칹��ĽṹӦ��������4��̼ԭ�ӵĽṹ������д������C��C��C��CHO��Ȼ���һ��̼ԭ�Ӻ�һ���ǻ��̶��������ϣ�����7�֣���ѡ2�ּ��ɡ���5���ɷ���ʽC5H10O2��֪A������ͬ���칹��Ӧ�DZ���һԪ������������������ˮ�⣬����������Է���������ͬ�Ļ������֪���������д��ķ���ʽΪC3H8O����ķ���ʽΪC2H4O2����������һ�ֵķ�������2������֪�����ĽṹΪ(CH3)2CH��OH���������ĽṹΪCH3COOCH(CH3)2����6�����ȸ�����Ϣ����ϼ��ɼ�λ�ã�Ȼ����ݶϼ��ɼ�λ����д�߾���Ľṹ��ʽ��
,��Ϊ�봼�ǻ����ڵ�̼ԭ��������ԭ�ӣ����Բ��ܷ�����ȥ��Ӧ����4������ʽΪC5H10O2����������3��̼ԭ�ӵ��л���ΪA��������A������ͬ�����Ų�����֧����ͬ���칹��ĽṹӦ��������4��̼ԭ�ӵĽṹ������д������C��C��C��CHO��Ȼ���һ��̼ԭ�Ӻ�һ���ǻ��̶��������ϣ�����7�֣���ѡ2�ּ��ɡ���5���ɷ���ʽC5H10O2��֪A������ͬ���칹��Ӧ�DZ���һԪ������������������ˮ�⣬����������Է���������ͬ�Ļ������֪���������д��ķ���ʽΪC3H8O����ķ���ʽΪC2H4O2����������һ�ֵķ�������2������֪�����ĽṹΪ(CH3)2CH��OH���������ĽṹΪCH3COOCH(CH3)2����6�����ȸ�����Ϣ����ϼ��ɼ�λ�ã�Ȼ����ݶϼ��ɼ�λ����д�߾���Ľṹ��ʽ��
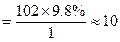 ����Oԭ�Ӹ���Ϊ2��Cԭ�Ӹ���
����Oԭ�Ӹ���Ϊ2��Cԭ�Ӹ���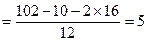 ������A�ķ���ʽΪC5H10O2��2����ͬ�ĺ�����������ȩ�������ǻ�����3��A��������Ӧ����B��֪��B�ķ���ʽΪC5H12O2�������������ǻ�������B�ĽṹΪ
������A�ķ���ʽΪC5H10O2��2����ͬ�ĺ�����������ȩ�������ǻ�����3��A��������Ӧ����B��֪��B�ķ���ʽΪC5H12O2�������������ǻ�������B�ĽṹΪ ����A�ĽṹΪ
����A�ĽṹΪ ,��Ϊ�봼�ǻ����ڵ�̼ԭ��������ԭ�ӣ����Բ��ܷ�����ȥ��Ӧ����4������ʽΪC5H10O2����������3��̼ԭ�ӵ��л���ΪA��������A������ͬ�����Ų�����֧����ͬ���칹��ĽṹӦ��������4��̼ԭ�ӵĽṹ������д������C��C��C��CHO��Ȼ���һ��̼ԭ�Ӻ�һ���ǻ��̶��������ϣ�����7�֣���ѡ2�ּ��ɡ���5���ɷ���ʽC5H10O2��֪A������ͬ���칹��Ӧ�DZ���һԪ������������������ˮ�⣬����������Է���������ͬ�Ļ������֪���������д��ķ���ʽΪC3H8O����ķ���ʽΪC2H4O2����������һ�ֵķ�������2������֪�����ĽṹΪ(CH3)2CH��OH���������ĽṹΪCH3COOCH(CH3)2����6�����ȸ�����Ϣ����ϼ��ɼ�λ�ã�Ȼ����ݶϼ��ɼ�λ����д�߾���Ľṹ��ʽ��
,��Ϊ�봼�ǻ����ڵ�̼ԭ��������ԭ�ӣ����Բ��ܷ�����ȥ��Ӧ����4������ʽΪC5H10O2����������3��̼ԭ�ӵ��л���ΪA��������A������ͬ�����Ų�����֧����ͬ���칹��ĽṹӦ��������4��̼ԭ�ӵĽṹ������д������C��C��C��CHO��Ȼ���һ��̼ԭ�Ӻ�һ���ǻ��̶��������ϣ�����7�֣���ѡ2�ּ��ɡ���5���ɷ���ʽC5H10O2��֪A������ͬ���칹��Ӧ�DZ���һԪ������������������ˮ�⣬����������Է���������ͬ�Ļ������֪���������д��ķ���ʽΪC3H8O����ķ���ʽΪC2H4O2����������һ�ֵķ�������2������֪�����ĽṹΪ(CH3)2CH��OH���������ĽṹΪCH3COOCH(CH3)2����6�����ȸ�����Ϣ����ϼ��ɼ�λ�ã�Ȼ����ݶϼ��ɼ�λ����д�߾���Ľṹ��ʽ��
��ϰ��ϵ�д�
�����Ŀ
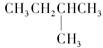 ��CH3CH2CH2CH3
��CH3CH2CH2CH3