��Ŀ����
18��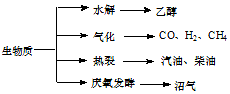 ��������Դ��һ����ȾС�Ŀ�������Դ�������ʵ���Ҫת��;������Ҫ������ͼ��
��������Դ��һ����ȾС�Ŀ�������Դ�������ʵ���Ҫת��;������Ҫ������ͼ����1�������й�˵����ȷ����abd
a���������ܣ�������������Դ��̫����
b������ά��ˮ���õ��Ҵ�����������
C���������ѽ��õ����͡����͵����ڴ�����
d����ֲ��ոѵȷ��ͻ�õ���������Ҫ�ɷ��Ǽ���
��2�����������ܻ�õ�CO��H2��������1��1����Ӧ����ԭ�������ʴ�100%���ϳɵ����ʿ�����cd��
a������ b���״� c����ȩ��HCHO�� d������
��3����֪������������¯�пɷ�����
C��s��+CO2��g���T2CO��g������H=+172kJ/mol
CH4��g��+H2O��g���TCO��g��+3H2��g������H=+206kJ/mol
CH4��g��+2H2O��g���TCO2��g��+4H2��g������H=+165kJ/mol
��C��s��+H2O��g���TCO��g��+H2��g������H=+131kJ/mol��
��4�����������ܻ�õ�CO��H2���������ϳ�Һ̬ȼ�ϼ״���ʵ���ã�5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��l����H=-1452.8kJ/mol��
��5����֪ϡ��Һ�У�1molH2SO4��NaOH��Һǡ����ȫ��Ӧʱ���ų�114.6kJ������д����ʾH2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽNaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
���� ��1��a������������Դ��̫���ܣ�
b���Ҵ���Դ����ά�أ�
c���������ָ�ɶ���������ɵ����ʣ�
d����������Ҫ�ɷ��Ǽ��飻
��2�����������غ㶨�ɺͻ��Ϸ�Ӧ�Ķ�����ص��жϣ���Ҫ�����÷�Ӧǰ��ԭ�ӵ�����䣬����Ŀ�ر��ֲ�������жϣ�
��3�����ø�˹���ɣ�����+��-�ڿɵá�H��
��4�������Ȼ�ѧ����ʽ����д������֪����ѧ�������뷴Ӧ�ȳ����ȣ���ע��������ʵľۼ�״̬�����
��5�������к��ȵĸ��ϡ��ǿ���ǿ�Ӧ����1molˮ���ų�����������к����Լ��к��ȵ��Ȼ�ѧ����ʽ��
��� �⣺��1��a�������ʱ���������Դ��̫���ܣ���A��ȷ��
b���Ҵ���Դ����ά�أ������������ܣ���B��ȷ��
c�����͡����͵����ڻ�����C����
d����������Ҫ�ɷ��Ǽ��飬��D��ȷ��
�ʴ�Ϊ��abd��
��2������ɫ��ѧ����ʵ�����ŷţ�����Ӧ���е�ԭ�������ʴﵽ100%��Ҳ����˵��Ӧ����һ���ǻ��Ϸ�Ӧ���ҷ�Ӧ������еĸ�ԭ����Ŀ�Ȳ��䣬CO��H2��һ�������°��ղ�ͬ�ı�����Ӧ���ɼٶ���Ӧʱ�Ļ�ѧ������֮��Ϊ1��1����ֻҪ��ѡ���еĻ�ѧʽ�ܻ�Ϊ��ʽ��CO��n��H2��n��������ȷ�ģ������ǻ����״���CH4O���ɱ�Ϊ��CO��1��H2��2����ȩ��CH2O���ɱ�Ϊ��CO��1��H2��1�����ᣨC2H4O2���ɱ�Ϊ��CO��2��H2��2��
�ʴ�Ϊ��cd��
��3����֪����C��s��+CO2��g��=2CO��g����H=172kJ/mol
��CH4��g��+H2O��g��=CO��g��+3H2��g����H=206kJ/mol
��CH4��g��+2H2O��g��=CO2��g��+4H2��g����H=165kJ/mol
Ϊ��C��s��+H2O��g��=CO��g��+H2��g������H1�ķ�Ӧ�ȣ����ø�˹���ɽ���+��-�ڿɵã�
��H=172kJ/mol+165kJ/mol-206kJ/mol=+131kJ/mol��
�ʴ�Ϊ��+131��
��4��5gCH3OH��������ȼ������CO2��Һ̬ˮ���ų�113.5kJ������64g��1molCH3OH��������ȼ������CO2��Һ̬ˮ���ų�1452.8kJ������
���Ȼ�ѧ����ʽΪ��2CH3OH��g��+3O2��g���T2CO2��g��+4H2O��l����H=-1452.8KJ��
�ʴ�Ϊ��2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��l����H=-1452.8kJ/mol��
��5��1mol H2SO4��Һ������ NaOH��Һ��ȫ��Ӧ���ų�114.6kJ��������������2molˮ�ų�114.6kJ����������Ӧ�ķ�Ӧ��Ϊ-114.6kJ/mol��
�к���Ϊ-57.3kJ/mol�����к��ȵ��Ȼ�ѧ����ʽ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
���� ���⿼�����Ȼ�ѧ����ʽ����д�����ͼ���Ӧ�ã���˹���ɵļ���Ӧ�õȣ��ѶȽϴ�ע��֪ʶ�Ļ��ۣ�


| A�� | �ŵ�ʱ��Na+��ʯīb��ʯīaǨ�� | |
| B�� | ���ʱʯīb����ӵ�Դ�������� | |
| C�� | ���ʱ��ÿ��23gNa���ɣ�����������ͨ��1mol���� | |
| D�� | �ŵ�ʱʯīb�Ϸ����ķ�ӦΪ��2Na++xS+2e-=Na2Sx |
| A�� | 1L 0.1mol/L �İ�ˮ����0.1NA��OH- | |
| B�� | ��״���£�2.24L�״��к���C-H������ĿΪ0.3NA | |
| C�� | 1mol CH3COOC2H5��NaOH��Һ����ȫˮ��õ��Ҵ�������ΪNA | |
| D�� | ��1molCl2ͨ��ˮ�У�HClO��Cl-��ClO-������֮��Ϊ2NA |
| A�� | �辧����а뵼�����ܣ���������ȡ���ά | |
| B�� | ������������Ư��ֽ����������ɱ������ | |
| C�� | SO2��NO2��CO2���ᵼ��������γ� | |
| D�� | ������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ |
| A�� | �����£�10 mL 0.2mol/L NH4NO3��Һ��10 mL 0.1mol/L NaOH��Һ��Ϻ�����pH=9.6����Һ�У�c��NO3-����c��NH4+����c��Na+����c��NH3•H2O����c��OH-����c��H+�� | |
| B�� | 0.1 mol/LNa2S��Һ�У�c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� | |
| C�� | ������a mL 0.1 mol/L KOH��b mL 0.1 mol/L HCN����Һ��Ϻ�pH��7������a��b��a��b | |
| D�� | 10 mL 0.1mol/L NaCl��Һ����������Ϊx��10mL 0.1mol/L CH3COONa��Һ����������Ϊy����x��y |
| A�� |  | |||||||
| B�� |  | |||||||
| C�� |  | |||||||
| D�� |
| |||||||
| A�� | �춡�������ԭ�Ӷ�λ��ͬһƽ���� | |
| B�� | ������ˮ��Ӧ��ȡ�屽���ڼӳɷ�Ӧ | |
| C�� | ����������������ˮ������Ϊ������ | |
| D�� | �һ��������һ�������6�� |
