��Ŀ����
11��0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol����1����A�ķ���ʽΪC6H12��
��2������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ
 ��
����3������A��ʹ��ˮ��ɫ���ڴ��������£���H2�����ӳɷ�Ӧ����ӳɲ���B���ⶨ�����к���4����������˴Ź�������ͼ��������壬��A�����еĽṹ��ʽΪCH3-C��CH3��=C��CH3��-CH3��
���� ��1��0.2molCxHy��1.2molCO2+1.2molH2O����ԭ���غ������
��2��A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣���ֻ��һ��H��Ϊ�����飻
��3������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳ����ɣ���ӳɲ��ᆳ�ⶨ�������к���4������
���ɵ������ṹʽΪ ��
�� ����ӳɲ���B���ⶨ�����к���4����������˴Ź�������ͼ��������壬ӦΪ
����ӳɲ���B���ⶨ�����к���4����������˴Ź�������ͼ��������壬ӦΪ ���Դ�ȷ��A�Ľṹ��ʽ��
���Դ�ȷ��A�Ľṹ��ʽ��
��� �⣺��1��0.2mol��A����������ȫȼ�պ�����CO2��H2O��1.2mol����A�����к���C��Hԭ�����ֱ�Ϊ��N��C��=$\frac{1.2mol}{0.2mol}$=6��n��H��=$\frac{1.2mol��2}{0.2mol}$=12��A����ʽΪ��C6H12��
�ʴ�Ϊ��C6H12��
��2��C6H12ֻ��1�������Ͷȣ�����A����ʹ��ˮ��ɫ������Ϊ������������������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�˵���������ֻ��1�ֵ�ЧH���ʸ��л����ǻ����飬�ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
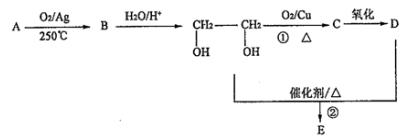
��3����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4������˵�����к���C=C�����к���4��������3�֣���̼�ܽṹΪ���٢ڢ۴��ɷֱ�ͬʱ����˫���� �������еĽṹ��ʽΪ����CH3��3C-CH=CH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2����B�����к˴Ź�������ͼ��������壬˵��B���Ӿ��жԳƽṹ������������B�ṹ��ʽΪ��CH3-CH��CH3��CH��CH3��-CH3��������Ϊ��2��3-�������飬��A�Ľṹ��ʽΪ��CH3-C��CH3��=C��CH3��-CH3��
�������еĽṹ��ʽΪ����CH3��3C-CH=CH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2����B�����к˴Ź�������ͼ��������壬˵��B���Ӿ��жԳƽṹ������������B�ṹ��ʽΪ��CH3-CH��CH3��CH��CH3��-CH3��������Ϊ��2��3-�������飬��A�Ľṹ��ʽΪ��CH3-C��CH3��=C��CH3��-CH3��
�ʴ�Ϊ��CH3-C��CH3��=C��CH3��-CH3��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ��������ճ����л���ṹ������Ϊ���ؼ������������ͬ���칹����жϣ�ע��������ʵ������жϿ��ܾ��еĽṹ��


��1��KMnO4ϡ��Һ��һ�ֳ����������������������������������Ƶ���AD������ţ�
A��˫��ˮ B��75%�ƾ� C������ D��84����Һ��NaClO��Һ��
��2���ٺ�ɫ��������ˮ��ʱΪ��߽������ʣ������õĴ�ʩΪ���ȡ�������������顢���裨����������ɣ���
������ˮϴ���պ������һ�ֺ�ɫ�Ļ��������a�еõ��ۿ����Ҫ�ɷ���K2MnO4��KCl���ù����з�����Ӧ�Ļ�ѧ����ʽΪ��3MnO2+KClO3+6KOH $\frac{\underline{\;����\;}}{\;}$3K2MnO4+KCl+3H2O��
��ͼ�в���A��һ��������ˮ�ĺ�ɫ���壬�仯ѧʽΪ��MnO2��
��3���ⶨKMnO4��Ʒ�Ĵ��ȿ��ñ�Na2S2O3��Һ���еζ���
������250 mL 0.100 0 mol•L-1��Na2S2O3��Һ����Ҫʹ�õIJ����������ձ�����ͷ�ιܡ���Ͳ�Ͳ����� ��250mL����ƿ ��
��ȡ�����Ƶõ�KMnO4��Ʒ0.700 0 g���ữ����0.100 0 mol•L-1��Na2S2O3��Һ���еζ����ζ����յ��¼ʵ������Na2S2O3��Һ��������ظ�����ڣ�����ƽ��ʵ�����������
| ʵ����� | 1 | 2 | 3 |
| ����Na2S2O3��Һ���/mL | 19.30 | 20.98 | 21.02 |
��0.100 0 mol•L-1��Na2S2O3��Һʢװ�ڼ�ʽ�����ʽ����ʽ�����ζ����н��еζ��������KMnO4��Ʒ�Ĵ���75.84%��
| A�� | X��Y��Ũ�Ȳ��ٱ仯 | |
| B�� | ��λʱ������amolX��ͬʱ����3amolY | |
| C�� | X��Y��Z�ķ�����֮��Ϊ1��3��2 | |
| D�� | Xռ������������������� |
| A�� | ������Һ�����Ϊ22.5L | |
| B�� | ����Һ���ʵ���Ũ��Ϊ10.00mol•L-1 | |
| C�� | �����������ݣ�����ø���Һ���ʵ���Ũ�� | |
| D�� | ����Һ�����ʵ�������������Һ���ܶ�δ֪������� |
| A�� | C2H4��C4H8 | B�� | ����ͱ�ϩ�� | ||
| C�� |  �� �� | D�� | ���Ǻ���ѿ�� |
| A�� | ���� | B�� | ���� | C�� | ��֬ | D�� | ������ |
��1�����ʵ���������ǿ ��2�����ʵ���ɫ����
��3����̬�⻯����ȶ�����ǿ ��4�����ʵķе�����
��5�������ӵĻ�ԭ����ǿ��
| A�� | ��1����2 �� ��3�� | B�� | ��2����3����4�� | C�� | ��2����4����5�� | D�� | ��1����3����5�� |
 ��
��