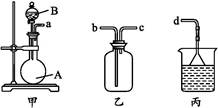
��Ŀ����
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ��������£�
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ����ȥ�����ۣ�������____________________��
��2��������ҺA��pH�ɲ���Zn(OH)2������Ϊ�Ƶ�ZnO����������������_____��ϴ�ӡ�_____��
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2����ԭ����________________��
��4�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������д������������K2Cr2O7��Fe2+��Ӧ�����ӷ���ʽ ��K2Cr2O7����ԭΪCr3+����
��5����������Ũ��Ϊ0.01000 mol·L-1��K2Cr2O7����Һ250mL��Ӧȷ��ȡK2Cr2O7 g��������λ��Ч���֣���֪M(K2Cr2O7)="294.0" g·mol-1�������Ƹñ���Һʱ����������һ����Ҫ�õ����� ���ñ�ű�ʾ����
�ٵ�����ƽ ���ձ� ����Ͳ �ܲ����� ��250 mL����ƿ ��ͷ�ι� ��������ƽ
��6��������K2Cr2O7����Һʱ�����ӿ̶��ߣ���ⶨ���_______���ƫ����ƫС�����䡱����ͬ�����ζ������У����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ζ������________��
��1���ܽ��п�� ��2�����ˡ����� ��3��N2�����£���ֹFe2+������
��4��6Fe2+ + Cr2O72- + 14H+ = 6Fe3+ + 2Cr3+ + 7H2O ��5��0.7350 �ۢ� ��6��ƫС ƫ��
���������������1��NaOH��Һ����п������Ӧ��������NaOH��Һ�����Ͼɶ�п��Ƥ����ȥ�����ۣ��������ܽ��п�㡣��2����Ӧ����Zn(OH)2���������ȷֽ��������п��ˮ������Ϊ�Ƶ�ZnO���������������ǹ��ˡ�ϴ�ӡ����ա���3��������ѧ���ʲ����ã������ֶ��Ի����пɷ�ֹFe2+����������4����Ӧ�����ַ���ʽ�ǣ�6Fe2+ + Cr2O72- + 14H+ = 6Fe3+ + 2Cr3+ + 7H2O ��5��m=n·M="0.01mol/L��0.25L��294.0" g/mol=0.7350g.�������ʵ���Ũ�ȵ���Һ�õ��������Тٵ�����ƽ ���ձ� ����Ͳ �ܲ����� ��250 mL����ƿ ��ͷ�ιܡ���6��������K2Cr2O7����Һʱ�����ӿ̶��ߣ�������Һ�����ƫС����K2Cr2O7����ҺŨ��ƫ�������ֱ���Һ�ⶨ�����ĵı���Һ��ƫС���ƫС���ζ������У����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ�����ĵı���Һ���������ƫ����ζ������ƫ��
���㣺����п�Ļ�ѧ���ʼ��ζ������������ʵ���Ũ�ȵ���Һ�����Ƶ�֪ʶ��

�����й�ʵ��ԭ����ʵ�������ȷ����
| A���ø���pH��ֽ�ⶨij������ˮ��pH |
| B����ͼ1װ���ܳ�ȥ�����л��е���ϩ���õ����������� |
| C����ͼ2װ������֤HC1������ˮ�е��ܽ��� |
| D����25mL��ʽ�ζ�����ȡ20.00 mL KMnO4��Һ |
���ж�������ȷ����( )
| A���ù㷺pH��ֽ���ij��Һ��pHΪ3.4 |
| B����10 mL ��Ͳ��ȡ8.8 mL NaOH��Һ |
| C����������ƽ����7.9 g NaCl���� |
| D������ʽ�ζ�����ȡ18.00 mL HCl |
��һ��ʵ������Na2CO3��10H2O����500ml 0.10mol��L-1��Na2CO3��Һ����ղ���ش��������⣺
��1������ʵ��Ҫ�������
| Ӧ��ȡNa2CO3��10H2O������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ�������������� |
| | | |
��2������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ��) ��
A��������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ�У���
B����������ƽȷ��������Na2CO3��10H2O�������������ձ��У��ټ�������ˮ���ò���������������ʹ���ܽ�(��Ҫʱ�ɼ���)��
C��������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3�������������������������ҺŨ�Ƚ��к�Ӱ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)?��û�н���A���� ����������ˮʱ���������˿̶� ��������ʱ���ӿ̶��� ��
��������Ȫʵ����һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ��Ը�����ͼ���ش��������⣺

��1����ͼA����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣��������в������γ���Ȫ���ǣ� ����
A��HCl��H��O B��Cl2��NaOH��Һ
C��HCl������ D��CO����NaOH��Һ
��2����ͼB����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ����� Ȫ���ǣ� ��
A��Cu��ϡ���� B��NaHCO����NaOH��Һ
C��CaCO����ϡ���� D��NH4HCO����ϡ����
��3����ͼB����ƿ���һˮ�ۣ���ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʲ������ǣ� ����
A��Ũ���� B����ʯ�� C������� D���ռ�
��4���Ƚ�ͼA��ͼB����װ�ã��Բ�����Ȫ��ԭ����������ͼA��_______�ϲ���ƿ��ѹǿ�������г�����������Ȫ����ɽ������ԭ��������________����ͼA��ͼB��װ�õ�ԭ�����ơ�
��ѧʵ���У�ͬһ��װ�ÿ������ڲ�ͬ��ʵ�飬����ͼ��ʵ��װ�ã�B�п�Ϊ�����Һ�壬�ɼ��ȣ���
��1����A��ΪŨ���ᣬB��Ϊ������ع��壬D��Ϊʯ����Һ����D������Ϊ________________________��
��2����A��Ϊ������Ũ���ᣬB��Ϊͭ���ʣ�D����NaOH����������������壬��д��D���������ķ�Ӧ�Ļ�ѧ����ʽ________________________________________________��
��3����A��ΪŨH2SO4��B��ΪNaCl���壬 D����Na2S��Na2SO3�Ļ����Һ����Һ©�����ȣ�D�г��ֻ�ɫ�������г�������ζ�������ݳ�����D��n(Na2S)��n(Na2SO3)�������������______________________________��
��4����װ�ÿ���ģ�ⰱ��ƴ��D��Ϊ����ʳ��ˮ������ȡ________����ͨ��D�������ͣ�����ȡ_______����Ҳͨ��D�У���ʱ���Կ�����Һ���о�����������д���÷�Ӧ�Ļ�ѧ����ʽ___________________________________________________��
��5����������װ�ã�����ʵ�鲻����ʵ�ֵ���_____________________
| A��֤��̼��ȱ��ӵ�����ǿ | B��֤�������������Ա���ǿ |
| C�����Ҵ��Ʊ���������ϩ | D���Ʊ����ռ��������� |
������һ����Ҫ���Լ������������ò���̽��Ũ�ȶԷ�Ӧ����Ӱ���ʵ�顣
��1��Ϊ֤��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬���н̿��顶��ѧ��Ӧԭ�������������ʵ�飺ȡ��֧�Թܣ�������4mL0.01mol��L-1��KMnO4������Һ���ֱ������м���0.1 mol��L-1��0.2 mol��L-1 H2C2O4��Һ2mL����¼��Һ��ɫ����ʱ�䡣
ʵ���з�����Ӧ�����ӷ���ʽΪ�� ��
Ԥ�������ǣ�
����Һ����ɫ�� ɫ��Ϊ ɫ��
�����м��� mol��L-1H2C2O4����֧�Թ��е���Һ�ȱ�ɫ��
Ȼ��ʵ���������������⡣ʵ�������ɫ���ӣ�����ɫ�Ȼ��������ӿ죻���������Dz���Ũ�ȴ�Ӧ����ȴ������
��ʵ���ܷ���Ϊ����ʵ���о�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죿���˵������������ģ�ijУһ�о�С��Դ˽�����̽�������������ǵ�ʵ�鱨���һ���֣�
��1�����鰲�ż����
| ʵ�� ��� | A��KMnO4��ҺŨ��/mol��L-1�� | B��������ҺŨ��/mol��L-1�� | C��������ҺŨ��/mol��L-1�� | ��ɫʱ��/s |
| 1 | 3 | 3 | 1 | 336 |
| 2 | 1 | 2 | 3 | 82 |
| 3 | 3 | 1 | 3 | 76 |
| 4 | 1 | 3 | 2 | 133 |
| 5 | 2 | 3 | 3 | 102 |
| 6 | 3 | 2 | 2 | 156 |
| 7 | 2 | 2 | 1 | 300 |
| 8 | 2 | 1 | 2 | 115 |
| 9 | 1 | 1 | 1 | 200 |
Ӧ��SPSS16.0���������������з��������������±�
��2��������ˮƽ�����ݴ������
| | A��KMnO4��Һ�� | B��������Һ�� | C��������Һ�� | ||||||
| Ũ��/mol��L-1 | 0.005 | 0.010 | 0.015 | 0.1 | 0.5 | 0.9 | 6 | 12 | 18 |
| ƽ����ɫʱ��/s | 138.3 | 172.3 | 189.3 | 130.3 | 179.3 | 190.3 | 278.7 | 134.7 | 86.7 |
��2���ɱ�2��֪���������У� ��Ũ�ȣ�ѡ�A��B��C�����¿�ͬ���Է�Ӧ����Ӱ���������� ��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�첻������
��3���ɱ�2��֪�����������Ũ��Ϊ mol��L-1������Ũ��Ϊ mol��L-1ʱ����Ӧ��졣������A��B�Ľ�����ʵ����������ȷ����
��������ʵ��������С��ͬѧ����̽�������Ũ��������Ӱ�챾��Ӧ���ʵģ��������ʵ������
��3����ͬ����Ũ���µ���ɫʱ��
| c(H2SO4)/mol��L-1 | 18 | 16 | 14 | 12 | 10 | 8 | 6 |
| ��ɫʱ��/s | 67 | 83 | 90 | 103 | 129 | 146 | 200 |
��4�����ݿ���ʵ��ĺ���ʱ�䣬��ѡ��Һ����ɫʱ��ԼΪ1���Ӻ�2���ӵ�������Һ������ʱ�����Ũ��Ϊ mol��L-1�� mol��L-1����Ҳ�����ڹ۲���������Ӧ���ʵIJ��졣
���ۣ����������Ը��������Һ�ķ�Ӧ������Ϊ����ʵ��̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졣