��Ŀ����
������Һ�����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ����
A��0.1mol��L-1NaHCO3��Һ��0.1 mol��L-1NaOH��Һ�������ϣ�������Һ�У�
c��Na������c��CO32������c��HCO3������c��OH����
B��20mL0.1 mol��L-1CH3COONa��Һ��10mL0.1 mol��L-1HCl��Һ��Ϻ�����ԣ�������Һ�У�
c��CH3COO������c��Cl������c��CH3COOH����c��H����
C�������£�pH��2��������pH��12�İ�ˮ�������ϣ�������Һ�У�
c��Cl������c��H������c��NH4������c��OH����
D��0.1 mol��L-1CH3COOH��Һ��0.1 mol��L-1NaOH��Һ�������ϣ�������Һ�У�
c��OH������c��H������c��CH3COOH��
1Lij��Һ�к��е��������±���
���� | Cu2+ | Al3+ | NO3- | Cl- |
���ʵ���Ũ�ȣ�mol/L�� | 1 | 1 | a | 1 |
�ö��Ե缫������Һ������·����3mole-ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ����
A��������Һ������ B��a=3
C����������1.5molCl2 D�����������Ľ�����ͭ����
��1����֪�����£�Ksp[Fe��OH��3]=4.0��10-38, ��FeCl3��Һ�м���NaHCO3��Һ�������������壬��Ӧ�����ӷ���ʽΪ��____________��������������Һ��pH����Ϊ4������Һ��Fe3+Ũ��Ϊ_________mol•L-1
��2�������£�Ũ�Ⱦ�Ϊ0.1mol•L-1����������������Һ��PH���±�
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
PH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
��������Һ�е������ӣ����H+������ǿ����__________�����ݱ������ݣ�Ũ�Ⱦ�Ϊ0.01 mol•L-1���������������Һ�ֱ�ϡ��100����PH�仯������___________�����ţ���
A��HCN B��HClO C��CH3COOH D��H2CO3
��3�� ����˵������ȷ����___ ___ ___ ������ţ�
A��ij�¶��´�ˮ�е�c��H+��=10-6����������
B����ϡ����ϴ��AgCl��������ˮϴ�����AgClС
C����ͬ�¶��£�0.1mol/LNH4Cl��Һ��NH4+��Ũ�ȱ�0.1mol/L��ˮ��NH4+��Ũ�ȴ�
D����ͬ�¶��£�PH��Ϊ8��Na2CO3��NaHCO3��Һ��ǰ�����ʵ���Ũ�ȴ�
E.��ʯ��ˮ�м���CaO���壬��Һ��Ca2+��OH-�����ʵ�������С



 2CO��g�������Է����У���÷�Ӧ�Ħ�H>0
2CO��g�������Է����У���÷�Ӧ�Ħ�H>0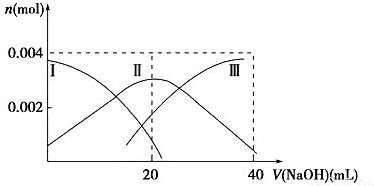
 H++OH- KW=10-14
H++OH- KW=10-14