��Ŀ����
��16�֣� ��1����֪��
��1����֪��
O2 (g) = O+2(g) + e- 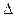 H1=" 1175.7" kJ��mol-1
H1=" 1175.7" kJ��mol-1 PtF6(g) + e- = PtF6-(g)
PtF6(g) + e- = PtF6-(g)
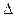 H2=" -" 771.1 kJ��mol-1
H2=" -" 771.1 kJ��mol-1 O2+ PtF6-(s) = O2+(g) + PtF6-
O2+ PtF6-(s) = O2+(g) + PtF6- 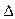 H3="482.2" kJ��mol-1
H3="482.2" kJ��mol-1  ��ӦO
��ӦO 2��g��+ PtF6 (g) = O2+PtF6- (s)
2��g��+ PtF6 (g) = O2+PtF6- (s) 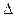 H="_____________" kJ��mol-1
H="_____________" kJ��mol-1
��2����C��S�γɵ�Һ̬������CS2��0.2mol/l CS2��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215k J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
��3����֪��������0.1mol/L��ij��H2A��pH=4�������Ϊ �ᣨ�ǿ������������H2A�ĵ��뷽��ʽΪ ������Һ����ˮ�������c(H+)= ��
��4��һ���¶��£������a������ b�����
�ٵ�����������ʵ���Ũ����ͬʱ��c(H+)��a b�����������������=������ͬ����
����pH��ͬ�������ͬ����������Һ�м�����������ۣ���Ӧ����ʱ����H2���������a b��
�۽�pH��ͬ�������ͬ����������Һ�ֱ��ˮϡ��100����������Һ��pHֵ��a b

����

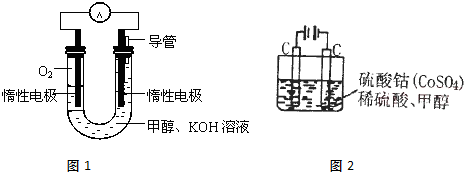
 ��2013?բ������ģ����ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
��2013?բ������ģ����ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
