��Ŀ����
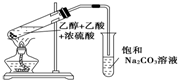 ʵ��������ͼ��ʾ��װ����ȡ��������
ʵ��������ͼ��ʾ��װ����ȡ����������1���ڴ��Թ�������һ���������Ҵ��������ٯ����Ļ��Һ�ķ�����
�ȼ���һ�������Ҵ���Ȼ�������Ũ����
�ȼ���һ�������Ҵ���Ȼ�������Ũ����
��2��Ũ�����������
��������ˮ��
��������ˮ��
�� ��3�������Թܢ��й۲쵽������֪��������������������
��ɫ��������ˮ���ܶȱ�ˮС��
��ɫ��������ˮ���ܶȱ�ˮС��
����4���Թܢ��б���Na2CO3��Һ��������
�ܽ��Ҵ�
�ܽ��Ҵ�
�������
�����
�����������������ܽ��
���������������ܽ��
��5������Na2CO3��Һ�ܷ�NaOH��Һ
����
����
��Ϊʲô��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH
CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH
�����û�ѧ����ʽ��ʾ����6���Թܢ��еĵ����ܲ��ܲ�����Һ�У�Ŀ���Ƿ�ֹ Na2CO3��Һ��������ɵ���������ԭ����
���Ȳ�����
���Ȳ�����
����7�����Թܢ��з��������������ʵ�������
��Һ
��Һ
����������1������Ҵ���Ũ����ķ������ȼ���һ�������Ҵ���Ȼ�������Ũ���ᣬ��ֹ��Һ�ɽ���
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
��3��ʵ��۲쵽��Һ�ֲ㣬��ɫ��״Һ�����ϵ�����
��4��ʵ�������ñ���̼������Һ��ȴ����������ԭ��ȥ������Ҵ��������������������ܽ�ȣ�����ˮ���ܶȣ�ʹ������ˮ�棬���ֲ����������ڷ��룮
��5�����ڼ�����������ȫˮ�⣮
��6����ֹ���ڼ��Ȳ��������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
��7�����뻥�����ܵ�Һ��ͨ���÷�Һ������
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
��3��ʵ��۲쵽��Һ�ֲ㣬��ɫ��״Һ�����ϵ�����
��4��ʵ�������ñ���̼������Һ��ȴ����������ԭ��ȥ������Ҵ��������������������ܽ�ȣ�����ˮ���ܶȣ�ʹ������ˮ�棬���ֲ����������ڷ��룮
��5�����ڼ�����������ȫˮ�⣮
��6����ֹ���ڼ��Ȳ��������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
��7�����뻥�����ܵ�Һ��ͨ���÷�Һ������
����⣺��1��Ϊ��ֹ��Һ�ɽ���Ӧ���ܶȴ��Һ����뵽�ܶ�С��Һ���У�
�ʴ�Ϊ���ȼ���һ�������Ҵ���Ȼ�������Ũ���ᣮ
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
�ʴ�Ϊ����������ˮ����
��3��ʵ��۲쵽ʢ�ű���̼������Һ�Թ�����Һ�ֲ㣬��ɫ��״Һ�����ϵ�����˵������������ɫ��������ˮ���ܶȱ�ˮС�ȣ�
�ʴ�Ϊ����ɫ��������ˮ���ܶȱ�ˮС�ȣ�
��4���Ʊ���������ʱ���ñ���̼������Һ����������������Ҫ���������������������ڱ���̼���ƣ��Ҵ���ˮ���ܣ������ܱ�̼�������գ����ڳ�ȥ���ʣ�
�ʴ�Ϊ���ܽ��Ҵ����к�������������������ܽ�ȣ�
��5����������������������Һ����ȫˮ�⣬��Ӧ����ʽΪCH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�ʴ�Ϊ�����ܣ�CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��6�����Ȳ����������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
�ʴ�Ϊ�����Ȳ����ȣ�
��7���Թ���Һ�岻���ܣ��ֲ㣬�����÷�Һ�������룮
�ʴ�Ϊ����Һ��
�ʴ�Ϊ���ȼ���һ�������Ҵ���Ȼ�������Ũ���ᣮ
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
�ʴ�Ϊ����������ˮ����
��3��ʵ��۲쵽ʢ�ű���̼������Һ�Թ�����Һ�ֲ㣬��ɫ��״Һ�����ϵ�����˵������������ɫ��������ˮ���ܶȱ�ˮС�ȣ�
�ʴ�Ϊ����ɫ��������ˮ���ܶȱ�ˮС�ȣ�
��4���Ʊ���������ʱ���ñ���̼������Һ����������������Ҫ���������������������ڱ���̼���ƣ��Ҵ���ˮ���ܣ������ܱ�̼�������գ����ڳ�ȥ���ʣ�
�ʴ�Ϊ���ܽ��Ҵ����к�������������������ܽ�ȣ�
��5����������������������Һ����ȫˮ�⣬��Ӧ����ʽΪCH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�ʴ�Ϊ�����ܣ�CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��6�����Ȳ����������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
�ʴ�Ϊ�����Ȳ����ȣ�
��7���Թ���Һ�岻���ܣ��ֲ㣬�����÷�Һ�������룮
�ʴ�Ϊ����Һ��
���������⿼�������������Ʊ�����Ŀ�ѶȲ���ע�����֪ʶ�Ļ�����������Ӧ������

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ
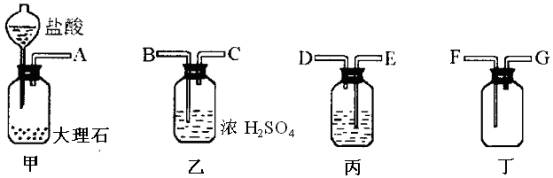
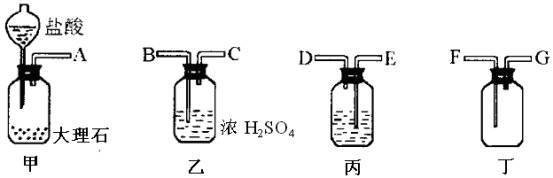
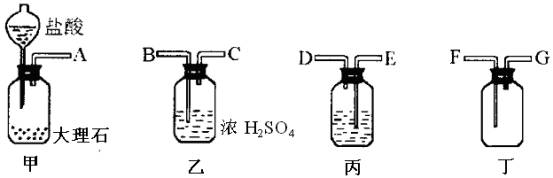
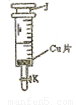

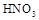 �У���ȡ������ϡ
�У���ȡ������ϡ