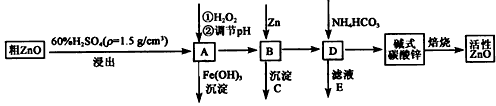
��Ŀ����
ZnO���п����ԣ�Ҳ����Ҫ����̥���Ӽ�����ҵ���ɴ�ZnO(��FeO��CuO)�Ʊ�����ZnO�������£�

��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�γ����������pH���±���
| ���� | ��ʼ������pH | ��ȫ������pH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.4 | 3.1 |
| Cu2+ | 5.2 | 6.7 |
| Zn2+ | 6.8 | 9 |
(1)ʵ��������98��H2SO4������100 mL60��ϡ��������ʹ�õIJ��������У���ͷ�ιܡ� �� �� ��
(2)д����A�м�H2O2�����ӷ���ʽ�� ��
(3)��A�п��Լ��� (д��ѧʽ)������ҺpH��Χ�� ֮�䣻����CΪ ��
(4)��ʽ̼��п[Zn3(OH)4CO3·H2O]�����Ʊ�����ZnO�Ļ�ѧ����ʽΪ ��
(5)����SO42-����BaCl2��Һ������ʱ��BaSO4��Ksp=1.08��10-10���ֽ�������� BaCl2��Һ��2.0��10��3mol/L��H2SO4��Һ��ϡ���Ҫ����BaSO4������ԭBaCl2��Һ����СŨ��Ϊ____________��
��1���ձ�������������Ͳ��3�֣�
��2����2Fe2++H2O2+2H+ = 2Fe3+2H2O����2�֣�
��3��ZnO 3.1��5.2֮�䡡��2�֣���Cu��2�֣�
��4��Zn3(OH)4CO3·H2O![]() 3ZnO+CO2��+3H2O��3�֣�
3ZnO+CO2��+3H2O��3�֣�

ZnO���п����ԣ�Ҳ����Ҫ����̥���Ӽ�����ҵ���ɴ�ZnO(��FeO��CuO)�Ʊ�����ZnO�������£�
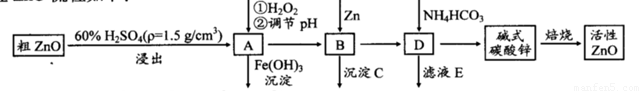
��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�γ����������pH���±���
|
���� |
��ʼ������pH |
��ȫ������pH |
|
Fe2+ |
6.4 |
8.4 |
|
Fe3+ |
2.4 |
3.1 |
|
Cu2+ |
5.2 |
6.7 |
|
Zn2+ |
6.8 |
9 |
(1)ʵ��������98��H2SO4������100 mL60��ϡ��������ʹ�õIJ��������У��ձ�����Ͳ�� �� �� ��
(2)д����A�м�H2O2�����ӷ���ʽ�� ��
(3)��A�п��Լ��� (д��ѧʽ)������ҺpH��Χ�� ֮�䣻����CΪ ��
(4)��ʽ̼��п[Zn3(OH)4CO3��H2O]�����Ʊ�����ZnO�Ļ�ѧ����ʽΪ ��
ZnO���п�����,Ҳ����Ҫ����̥���Ӽ�����ҵ���ɴ�ZnO����FeO��CuO���Ʊ�����ZnO�������£�
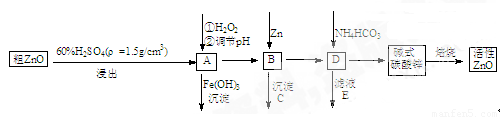
��֪����Һ��Fe2+ �� Fe3+�� Cu2+ �� Zn2+�γ����������pH���±�
|
���� |
��ʼ��������pH |
��ȫ������pH |
|
Fe2+ |
6.4 |
8.4 |
|
Fe3+ |
2.4 |
3.1 |
|
Cu2+ |
5.2 |
6.7[��Դ:Z*xx*k.Com] |
|
Zn2+ |
6.8 |
9 |
��1��ʵ��������98��H2SO4������ϡ��������ʹ�õIJ��������У���ͷ�ιܡ�_______��_______��_______��
��2����A�м�H2O2��Ŀ��֮һ��ʹ��ҺpH���ߣ�ʹFe3+������ȫ�������һ��Ŀ���� ����A�� (��ܡ����ܡ�)ʹFe2+ֱ�ӳ�����ȥ;
��3��ҪʹA��Һ˳����ΪB��Һ, ��Һ��pHӦ������ ; B�м���Zn�����ܽ���������Ũ������ ��
��4����д��ʽ̼��п�����Ʊ�����ZnO�Ļ�ѧ����ʽ .