��Ŀ����
 ��2012?���X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�
��2012?���X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ���ش��������⣺
��1��Y��Ԫ�����ڱ��е�λ��Ϊ
�ڶ����ڵڢ�A��
�ڶ����ڵڢ�A��
����2������Ԫ�ص�����������Ӧ��ˮ����������ǿ����
HClO4
HClO4
��д��ѧʽ�����ǽ�����̬�⻯�ﻹԭ����ǿ����H2S
H2S
��д��ѧʽ������3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������
O3��Cl2��
O3��Cl2��
��д�������������ʵĻ�ѧʽ������4��X2M��ȼ���ȡ�H=-a kJ?mol-1��д��X2Mȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
2H2S��g��+3O2��g��=2SO2��g��+2H2O��l������H=-2aKJ?mol-1
2H2S��g��+3O2��g��=2SO2��g��+2H2O��l������H=-2aKJ?mol-1
����5��ZX�ĵ���ʽΪ


NaH+H2O=NaOH+H2��
NaH+H2O=NaOH+H2��
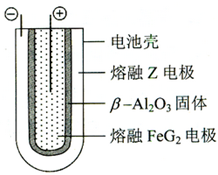
����6������״̬�£�Z�ĵ��ʺ�FeG2����ɿɳ���أ�װ��ʾ��ͼ���£�����Ӧԭ��Ϊ��2Z+FeG2
| ��� | �ŵ� |
Fe2++2e-=Fe
Fe2++2e-=Fe
�����ʱ��Na
Na
��д�������ƣ��缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ��-Al2O3
��-Al2O3
��������X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������XΪ����Ԫ�أ�����X��HԪ�أ�X��Zͬ���壬���γ����ӻ�����ZX��YΪ����Ԫ�أ���Zԭ����������Yԭ������������Z��NaԪ�أ�Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�����Y��OԪ�أ�M��SԪ�أ�G�Ƕ���������Ԫ�أ�����G��ClԪ�أ�������ϡ�����壩���ݴ˽��
����⣺X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������XΪ����Ԫ�أ�����X��HԪ�أ�X��Zͬ���壬���γ����ӻ�����ZX��YΪ����Ԫ�أ���Zԭ����������Yԭ������������Z��NaԪ�أ�Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�����Y��OԪ�أ�M��SԪ�أ�G�Ƕ���������Ԫ�أ�����G��ClԪ�أ�������ϡ�����壩��
��1��Y��OԪ�أ�Oԭ����2�����Ӳ㣬����������Ϊ6�����ڵڶ����ڵڢ�A�壬�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2���ǽ�����Խǿ������Ӧ����ۺ����������Խǿ���⼸��Ԫ�طǽ�������ǿ����ClԪ�أ���������ۺ������������ǿ���Ǹ�����HClO4���ǽ�����Խ������̬�⻯�ﻹԭ��Խǿ����ԭ����ǿ����̬�⻯�������� H2S���ʴ�Ϊ��HClO4��H2S��
��3��Y�ĵ���O3��G�ĵ���Cl2�������γɵ�ClO2�������������ʴ�Ϊ��O3��Cl2�ȣ�
��4��H2S��ȼ���ȡ�H=-a kJ?mol-1������ȼ���ȵĺ��壬H2Sȼ�յ��Ȼ�ѧ����ʽ������Ӧ������SO2����H2Sȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ��2H2S��g��+3O2��g��=2 SO2��g��+2H2O��l����H=-2aKJ?mol-1��
�ʴ�Ϊ��2H2S��g��+3O2��g��=2 SO2��g��+2H2O��l����H=-2aKJ?mol-1��
��5��ZXΪNaH���������ӻ���������������⸺���ӹ��ɣ�����ʽΪ ��Na��ˮ��Ӧ��������������������Ӧ��ѧ����ʽΪΪ��NaH+H2O=NaOH+H2����
��Na��ˮ��Ӧ��������������������Ӧ��ѧ����ʽΪΪ��NaH+H2O=NaOH+H2����
�ʴ�Ϊ�� ��NaH+H2O=NaOH+H2����
��NaH+H2O=NaOH+H2����
��6������״̬�£�Na�ĵ��ʺ�FeCl2����ɿɳ���أ���Ӧԭ��Ϊ��2Na+FeCl2
Fe+2NaCl�� �ŵ�ʱ��Ϊԭ��أ�ԭ��ص�����������ԭ��Ӧ��Fe2+�������ŵ�����Fe��������ӦʽΪ��Fe2++2e-=Fe�����ʱ��Ϊ���أ�����������ԭ������Na�缫�ӵ�Դ�ĸ������ɵ�ؽṹ��֪���õ�صĵ����Ϊ��-Al2O3��
�ʴ�Ϊ��Fe2++2e-=Fe��Na����-Al2O3��
��1��Y��OԪ�أ�Oԭ����2�����Ӳ㣬����������Ϊ6�����ڵڶ����ڵڢ�A�壬�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2���ǽ�����Խǿ������Ӧ����ۺ����������Խǿ���⼸��Ԫ�طǽ�������ǿ����ClԪ�أ���������ۺ������������ǿ���Ǹ�����HClO4���ǽ�����Խ������̬�⻯�ﻹԭ��Խǿ����ԭ����ǿ����̬�⻯�������� H2S���ʴ�Ϊ��HClO4��H2S��
��3��Y�ĵ���O3��G�ĵ���Cl2�������γɵ�ClO2�������������ʴ�Ϊ��O3��Cl2�ȣ�
��4��H2S��ȼ���ȡ�H=-a kJ?mol-1������ȼ���ȵĺ��壬H2Sȼ�յ��Ȼ�ѧ����ʽ������Ӧ������SO2����H2Sȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ��2H2S��g��+3O2��g��=2 SO2��g��+2H2O��l����H=-2aKJ?mol-1��
�ʴ�Ϊ��2H2S��g��+3O2��g��=2 SO2��g��+2H2O��l����H=-2aKJ?mol-1��
��5��ZXΪNaH���������ӻ���������������⸺���ӹ��ɣ�����ʽΪ
 ��Na��ˮ��Ӧ��������������������Ӧ��ѧ����ʽΪΪ��NaH+H2O=NaOH+H2����
��Na��ˮ��Ӧ��������������������Ӧ��ѧ����ʽΪΪ��NaH+H2O=NaOH+H2�����ʴ�Ϊ��
 ��NaH+H2O=NaOH+H2����
��NaH+H2O=NaOH+H2������6������״̬�£�Na�ĵ��ʺ�FeCl2����ɿɳ���أ���Ӧԭ��Ϊ��2Na+FeCl2
| ��� |
| �ŵ� |
�ʴ�Ϊ��Fe2++2e-=Fe��Na����-Al2O3��
������������Ԫ���ƶ�Ϊ���忼����Ԫ�ػ���������ʣ�����ȷ�ж�Ԫ���ǽⱾ��Ĺؼ���ע�⣨5����NaH����ʽ��д�⸺���ӵĵ���ʽ2�����ӳɶԣ����ֿܷ���

��ϰ��ϵ�д�
�����Ŀ
 ��2012?���ģ�⣩������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���ģ�⣩������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������


