题目内容
长征二号捆绑式火箭推进剂以肼(N2H4)作为燃料,NO2作为氧化剂,反应生成N2和水蒸气。已知:N2(g) + 2O2(g) =2NO2(g); △H=+67.7 kJ/mol
N2H4(g) + O2(g) = N2(g) + 2H2O(g); △H=-534 kJ/mol
下列关于肼和NO2反应的热化学方程式中,正确的是
- A.2N2H4(g) + 2NO2(g) = 3N2(g) + 4H2O(l);△H=-1135.7 kJ/mol
- B.N2H4(g) + NO2(g) = 3/2N2(g) + 2H2O(g);△H=-567.85 kJ/mol
- C.N2H4(g) + NO2(g) = 3/2N2(g) + 2H2O(l);△H=-1135.7 kJ/mol
- D.2N2H4(g) + 2NO2(g) = 3N2(g) + 4H2O(g);△H=+1135.7 kJ/mol
B
根据盖斯定律
①N2(g) + 2O2(g) =2NO2(g); △H=+67.7 kJ/mol
②N2H4(g) + O2(g) = N2(g) + 2H2O(g); △H=-534 kJ/mol
②—①/2可得:N2H4(g) + NO2(g) = 3/2N2(g) + 2H2O(g);△H=-567.85 kJ/mol
故答案为B
根据盖斯定律
①N2(g) + 2O2(g) =2NO2(g); △H=+67.7 kJ/mol
②N2H4(g) + O2(g) = N2(g) + 2H2O(g); △H=-534 kJ/mol
②—①/2可得:N2H4(g) + NO2(g) = 3/2N2(g) + 2H2O(g);△H=-567.85 kJ/mol
故答案为B

练习册系列答案
相关题目
近年,我国在航天事业上取得了令世界瞩目的成就,神舟飞船多次被长征系
列火箭送入太空。
(1)长征二号捆绑式火箭推进剂以联氨(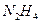 )作为燃料,
)作为燃料, 作为推进剂。
作为推进剂。
① 的主要作用是助燃,但其在工作时会产生红棕色气体
的主要作用是助燃,但其在工作时会产生红棕色气体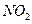 ,对环境会造
,对环境会造
成污染,为避免污染可使用下列 (填字母)代替之。
| A.液态氨 | B.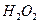 | C.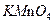 | D.液态氧 |
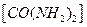 和次氯酸钠反应可以制取联氨(产物中
和次氯酸钠反应可以制取联氨(产物中同时有两种正盐),则该反应的离子方程式是 。
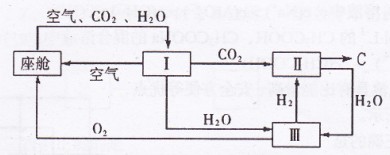
(2)飞船座舱内空气的更新过程如下图所示:
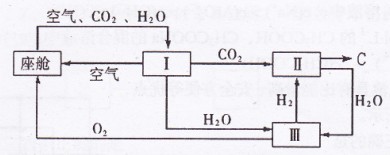
①座舱内空气更新过程可以循环利用的物质为
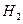 、
、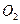 和 ;装置Ⅱ中
和 ;装置Ⅱ中发生反应的化学方程式为 。
②从装置I、Ⅱ、Ⅲ可看出
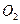 的来源,若宇航员每天消耗35mol
的来源,若宇航员每天消耗35mol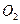 ,每天呼出
,每天呼出的气体中含18 mol
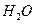 ,则呼出的气体中含
,则呼出的气体中含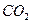 mol.
mol. 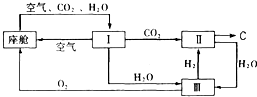 近年,我国在航天事业上取得了令世界瞩目的成就,神舟飞船多次被长征系列火箭送入太空.
近年,我国在航天事业上取得了令世界瞩目的成就,神舟飞船多次被长征系列火箭送入太空.