��Ŀ����
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�
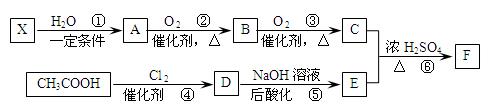
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
��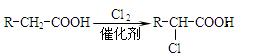 ��
��
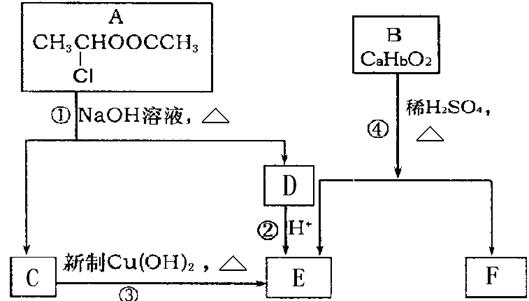
��C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��

��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
��
 ��
����C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
��1��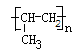 ��3�֣�
��3�֣�
��2����ԭ�ӡ��Ȼ���2�֣�
��3��2CH3CH2CH2OH +O2 2CH3CH2CHO+2H2O��3�֣�������Ӧ��1�֣�
2CH3CH2CHO+2H2O��3�֣�������Ӧ��1�֣�
CH3CH2COOH+ HOCH2COOH CH3CH2COOCH2COOH +H2O ��3�֣�
CH3CH2COOCH2COOH +H2O ��3�֣�
��4��CH3OOCCH2COOCH3��HCOOCH2CH2CH2OOCH��CH3COOCH2OOCCH3��3�֣�
��ֻҪд������һ�����ɣ�
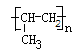 ��3�֣�
��3�֣���2����ԭ�ӡ��Ȼ���2�֣�
��3��2CH3CH2CH2OH +O2
 2CH3CH2CHO+2H2O��3�֣�������Ӧ��1�֣�
2CH3CH2CHO+2H2O��3�֣�������Ӧ��1�֣�CH3CH2COOH+ HOCH2COOH
 CH3CH2COOCH2COOH +H2O ��3�֣�
CH3CH2COOCH2COOH +H2O ��3�֣���4��CH3OOCCH2COOCH3��HCOOCH2CH2CH2OOCH��CH3COOCH2OOCCH3��3�֣�
��ֻҪд������һ�����ɣ�
��

��ϰ��ϵ�д�
�����Ŀ
 ƽ��2CH3CHO+O2
ƽ��2CH3CHO+O2 2CH3COOH������AΪ��Ҫԭ�Ϻϳ�E��E��һ������ζ���л����ϳ�·
2CH3COOH������AΪ��Ҫԭ�Ϻϳ�E��E��һ������ζ���л����ϳ�· ������ͼ��ʾ��
������ͼ��ʾ��
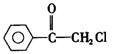 �� ��ش��������⣺
�� ��ش��������⣺ ��
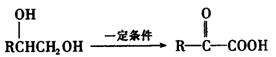
��
 ����M��F��ת����ϵ���£�
����M��F��ת����ϵ���£�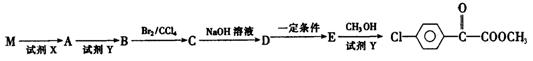
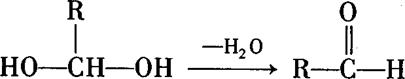
 ��壻E�к��еĹ����ŵ������� ��
��壻E�к��еĹ����ŵ������� ��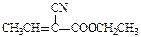 ���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·�����£�
���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·�����£�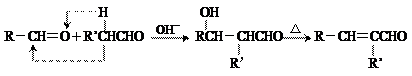
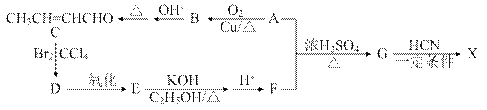
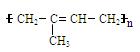
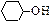 )�ϳɼ���ȩ(
)�ϳɼ���ȩ(  )������Ӧ�Ļ�ѧ����ʽ��
)������Ӧ�Ļ�ѧ����ʽ��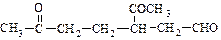 ��A���������õ��IJ���B���仯ѧʽΪC10H20�������ں�����Ԫ�������Ƶ�A�Ľṹ��ʽ��
��A���������õ��IJ���B���仯ѧʽΪC10H20�������ں�����Ԫ�������Ƶ�A�Ľṹ��ʽ��
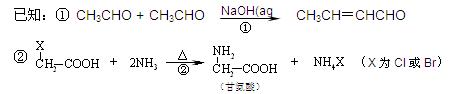
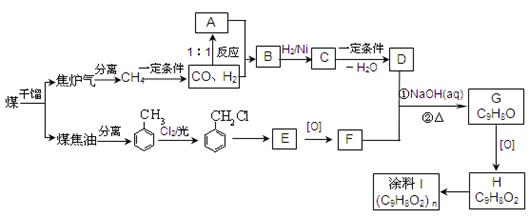
 H�ĺϳ�·�߲�ע����Ӧ������
H�ĺϳ�·�߲�ע����Ӧ������ ���������ױ����������ױ���һ����Ҫ���л�����ԭ�ϣ��������ϳɶ�����Ҫ���л���Լױ�Ϊ��ʼԭ�Ϻϳɰ�˾ƥ�ֵ��л����ת����ϵͼ�����ֲ���ϳ�·�ߡ���Ӧ������ȥ�����¡�
���������ױ����������ױ���һ����Ҫ���л�����ԭ�ϣ��������ϳɶ�����Ҫ���л���Լױ�Ϊ��ʼԭ�Ϻϳɰ�˾ƥ�ֵ��л����ת����ϵͼ�����ֲ���ϳ�·�ߡ���Ӧ������ȥ�����¡�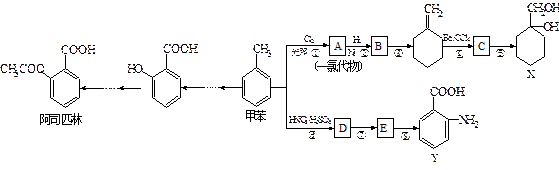 ��ش��������⣺
��ش��������⣺
