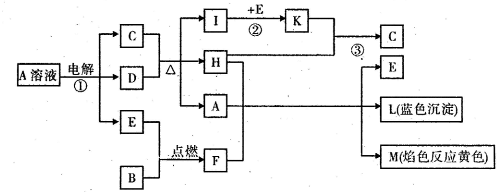
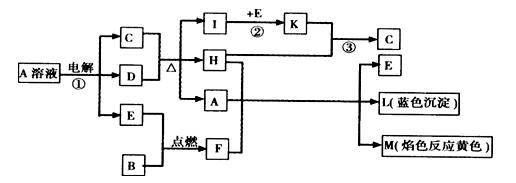
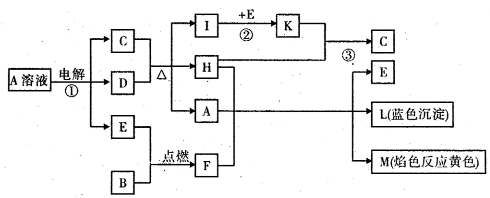
��Ŀ����
��֪B�dz����������ʣ�EΪ�����ǽ������ʣ�H������Ϊ��ɫҺ�壬C��Ũ��Һ�ڼ���ʱ����D��Ӧ���������п�ͼ��ʾ���Իش�
(1)д����ѧʽ��A____________��E______________��L______________��
(2)��Ӧ�ٵ����ӷ���ʽ��___________________________________��
(3)��Ӧ�ڣ���ҵ�ϲ�ȡ�ķ�Ӧ������________________________��
(4)��Ӧ�ۣ���ҵ�ϲ�ȡ�IJ�������Kֱ����H��Ӧ��ԭ����__________________��
(5)ÿ����1 mol K�� ��Ӧ�ų�98.3 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ��
��Ӧ�ų�98.3 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ��
___________________________________________ ________________________��
________________________��
(1)A��CuSO4 (1��) E��O2 (1��) L��Cu(OH)2 (1��)
(2) 2Cu2+ + 2H2O 2Cu + O2��+ 4H+ (3��)
2Cu + O2��+ 4H+ (3��)
(3) ��ѹ��������400�桫500��(���ѹ������������Ҳ��) (2��)
(4) �����������������SO3������ (2��)
(5) SO2(g) + 1/2O2(g) 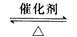 SO3(g)����H =�C98.3 kj/mol (3��)
SO3(g)����H =�C98.3 kj/mol (3��)
����

��ϰ��ϵ�д�
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ