��Ŀ����
����Ŀ����1��CuSO4��ˮ��Һ�� ����ᡱ�����С�������� �ԣ�ʵ����������CuSO4����Һʱ������CuSO4���������������У�Ȼ����������ˮϡ�͵������Ũ�ȣ��� ����ٽ����������ơ�����ˮ�⡣
��2����ĭ������е���Ҫ�ɷ���Al2(SO4)3��NaHCO3��Һ����Ӧ�����ӷ���ʽΪ
��3�������ӷ���ʽ��ʾ������ˮԭ��_________________
��4������Ag2S(s)![]() 2Ag+(aq)+ S2-(aq)����Ksp=____________��
2Ag+(aq)+ S2-(aq)����Ksp=____________��
��5������˵������ȷ����__________��
A����ϡ����ϴ��AgCl��������ˮϴ�����AgClС��
B�����ʵ��ܽ�����¶ȵ����߶����ӣ������ʵ��ܽⶼ�����ȵģ�
C������Al(OH)3(s)![]() Al(OH)3(aq)
Al(OH)3(aq)![]() Al3+��3OH-��ǰ��Ϊ�ܽ�ƽ�⣬����Ϊ����ƽ�⣻
Al3+��3OH-��ǰ��Ϊ�ܽ�ƽ�⣬����Ϊ����ƽ�⣻
D����ȥ��Һ�е�Mg2+����OH-����Mg2+����CO32-Ч���ã�˵��Mg(OH)2���ܽ�ȱ�MgCO3��
E��������Ӧ�г��ӹ����ij���������Ŀ����ʹ������ȫ��
���𰸡���1������ƣ���2��Al3++3HCO3-=Al(OH)3![]() +3CO2
+3CO2![]() ;
;
��3��Al3++3H2O![]() Al(OH)3(���壩+3H+;��4��
Al(OH)3(���壩+3H+;��4��![]() ;��5��BD
;��5��BD
��������
�����������1��CuSO4��ǿ�������Σ�ˮ��Һ�����ԣ�ʵ����������CuSO4����Һʱ��Ϊ����ˮ�⣬����CuSO4���������������У�Ȼ����������ˮϡ�͵������Ũ�ȡ���2��Al2(SO4)3��NaHCO3��Һ����˫ˮ�ⷴӦ����Ӧ�����ӷ���ʽΪAl3++3HCO3-=Al(OH)3![]() +3CO2
+3CO2![]() ;��3��������ˮ�����������������壬������ˮԭ�� Al3++3H2O
;��3��������ˮ�����������������壬������ˮԭ�� Al3++3H2O![]() Al(OH)3(���壩+3H+����4������Ag2S(s)
Al(OH)3(���壩+3H+����4������Ag2S(s) ![]() 2Ag+(aq)+ S2-(aq)����Ksp=
2Ag+(aq)+ S2-(aq)����Ksp= ![]()

����Ŀ���������⡿����ȩ������ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶȣ��ڼ��������·����绯��Ӧ�����Ʊ�����ȩ����ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶȣ��������ᡣ��Ӧԭ�����£�
2C6H5CHO+NaOH![]() C6H5CH2OH+C6H5COONa
C6H5CH2OH+C6H5COONa
C6H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
������������������±���
����ȩ | ���״� | ������ | �� | |
�е�/�� | 178 | 205 | 249 | 80 |
�۵�/�� | 26 | -15 | 122 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ���������£�

��1���ڢ�����������1Сʱ����ͼ1�������м��Ȼ�Ϲ̶�װ��Ϊ������
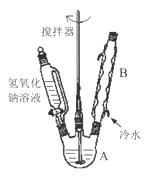
����A������Ϊ_______����������B��Ϊ����C��Ч������B��˵��ԭ��_______��
��2���������йط�Һ©����ʹ�ò���ȷ����_______
A.��Һ©����ʹ��֮ǰ��������Ƿ�©ˮ
B.��Һ©���ڵ�Һ�岻�ܹ��࣬����������
C.�����Һ©����������̨�Ͼ��ã��ֲ���������������з�Һ
D.��Һʱ���²�Һ�����������ر������������ձ��ٴ�����ʹ�ϲ�Һ������
��3�����������÷�ˮԡ���������ٽ��в����ܣ���ͼ2�����ռ�______�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ_____________��
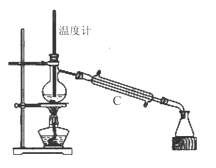
��4������ʱ����ͼ3���ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��_____��ϴ�����ϲ����ľ��塣������ɺ���������ˮ�Ծ������ϴ�ӣ�ϴ��Ӧ____________��
��5���õ�����ƽȷ��ȡ0.2440g����������ƿ�м�100mL����ˮ�ܽ⣨��Ҫʱ���Լ��ȣ�������0.1000mol/L�ı�����������Һ�ζ��������ı�����������Һ19.20mL��������Ĵ���Ϊ_____%��