��Ŀ����
����X��Y��Z������ת����ϵ

��ش��������⣺
(1)��X��Y��Z��Ϊ����Ԫ�صĻ����M�Ļ�ѧʽ������_____________________��
(2)��M�ǵڢ�A��ijԪ�ص�����������MΪ���壬�������ӻ�����X���������Ӿ�����10�����ӣ�Y��Һ��5������Ũ�ȵĴ�С˳��Ϊ��_________________��
(3)��X��һ�ֻ���ɫ���壬M��һ�ֳ���������д��Z��Y�����ӷ���ʽ��__________��
(4)��X��Y��Z�ж�������Ԫ�أ���֪���������Ȼ�ѧ����ʽ��
Xת��ΪY��2H2S(g)+O2(g)��2S(s)+2H2O(1) �� ��H =��Ql kJ/mol��
Xת��ΪZ��2H2S(g)+3O2(g)��2SO2(g)+2H2O(1)�� ��H =��Q2 kJ/mol��
��д��X��Z��Ӧת��ΪY���Ȼ�ѧ����ʽ��______________________��
(5)��X��Y��Z�о�����NԪ�أ����ڳ�ѹ�°��������ú���ϡ�͵�Y���壬�ֱ�ͨ�� һ�����ȵ�570��ĵ����У����ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫�����ɺϳ�X����(װ����ͼ)�����ڵ�ⷨ�ϳ�X�ĵ�����______ (��ܡ����ܡ�)��ˮ���������Һ���ܼ����ٵ缫A�ǵ��ص�______��(�����������)���ü��ϵĵ缫��ӦʽΪ��____________________��
(1)��X��Y��Z��Ϊ����Ԫ�صĻ����M�Ļ�ѧʽ������_____________________��
(2)��M�ǵڢ�A��ijԪ�ص�����������MΪ���壬�������ӻ�����X���������Ӿ�����10�����ӣ�Y��Һ��5������Ũ�ȵĴ�С˳��Ϊ��_________________��
(3)��X��һ�ֻ���ɫ���壬M��һ�ֳ���������д��Z��Y�����ӷ���ʽ��__________��
(4)��X��Y��Z�ж�������Ԫ�أ���֪���������Ȼ�ѧ����ʽ��
Xת��ΪY��2H2S(g)+O2(g)��2S(s)+2H2O(1) �� ��H =��Ql kJ/mol��
Xת��ΪZ��2H2S(g)+3O2(g)��2SO2(g)+2H2O(1)�� ��H =��Q2 kJ/mol��
��д��X��Z��Ӧת��ΪY���Ȼ�ѧ����ʽ��______________________��
(5)��X��Y��Z�о�����NԪ�أ����ڳ�ѹ�°��������ú���ϡ�͵�Y���壬�ֱ�ͨ�� һ�����ȵ�570��ĵ����У����ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫�����ɺϳ�X����(װ����ͼ)�����ڵ�ⷨ�ϳ�X�ĵ�����______ (��ܡ����ܡ�)��ˮ���������Һ���ܼ����ٵ缫A�ǵ��ص�______��(�����������)���ü��ϵĵ缫��ӦʽΪ��____________________��

(1)NaOH��HCl(������������)
(2)c(Na+)>c(CO32-)>c(OH-)>c(HCO3-)>c(H+)
(3) 2Fe2++ Cl2== 2Fe3++ 2Cl-
(4)2H2S(g)+SO2(g)��3S(s)+2H2O(1) ��H =��(3Ql��Q2)/2 kJ/mol
(5)���ܣ�����N2+6e-+6H+��2NH3
(2)c(Na+)>c(CO32-)>c(OH-)>c(HCO3-)>c(H+)
(3) 2Fe2++ Cl2== 2Fe3++ 2Cl-
(4)2H2S(g)+SO2(g)��3S(s)+2H2O(1) ��H =��(3Ql��Q2)/2 kJ/mol
(5)���ܣ�����N2+6e-+6H+��2NH3

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
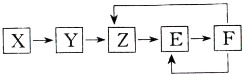 ���ֳ�������X��Y��Z��E��F������ͬһԪ��M����֪Y�ǵ��ʣ�Z��NO��һ������������һ������������������ת����ϵ�������ƶ��в��������ǣ�������
���ֳ�������X��Y��Z��E��F������ͬһԪ��M����֪Y�ǵ��ʣ�Z��NO��һ������������һ������������������ת����ϵ�������ƶ��в��������ǣ�������| A��X������һ���⻯�� | B��M�����ǽ��� | C��E������һ����ɫ���� | D��X��Y�����Ƿ�������ԭ��Ӧ |


 M3+ (aq) + 3OH-(aq) �����£�M(OH)3���ܶȻ�Ksp = 4.0 �� 10-38��ҪʹM3+����Ũ�Ƚ���10-5mol/L����Һ��PHӦ����_________�� (lg2=0.3,lg5=0.7)
M3+ (aq) + 3OH-(aq) �����£�M(OH)3���ܶȻ�Ksp = 4.0 �� 10-38��ҪʹM3+����Ũ�Ƚ���10-5mol/L����Һ��PHӦ����_________�� (lg2=0.3,lg5=0.7) 