��Ŀ����
������ʵĵ���ƽ�⡢�����ˮ��ƽ�����������ܽ�ƽ������ڻ�ѧƽ�⡣
��.��֪H2A��ˮ�д�������ƽ�⣺H2A=H����HA����HA��??H����A2����
��1��������NaHA��Һ��pH________(�����)��ԭ����_________________��
A������7 B����7
C������7 D����ȷ��
��2��ij�¶��£�����0.1 mol��L��1��NaHA��Һ����εμ�0.1 mol��L��1KOH��Һ����Һ������(���Ի�Ϻ���Һ������仯)����ʱ�û����Һ�е����й�ϵһ����ȷ����________��
A��c(H��)��c(OH��)��1.0��10��14
B��c(Na��)��c(K��)��c(HA��)��2c(A2��)
C��c(Na��)��c(K��)
D��c(Na��)��c(K��)��0.05 mol��L��1
��3����֪������H2A�ĸ���(CaA)�ı�����Һ�д�������ƽ�⣺CaA(s)??Ca2��(aq)��A2��(aq)����H��0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��________��
A�������¶� B�������¶�
C������NH4Cl���� D������Na2A����
��.����Cr2O72-�ķ�ˮ���Խϴ�ij������ˮ�к�5.0��10��3 mol��L��1��Cr2O72-��Ϊ��ʹ��ˮ���ŷŴ�꣬�������´�����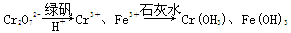
(1)�÷�ˮ�м����̷���H����������Ӧ�����ӷ���ʽΪ____________________��
(2)��������ķ�ˮ�в�����c(Fe3��)��2.0��10��13 mol��L��1���������Cr3����Ũ��Ϊ________��
(��֪��Ksp[Fe(OH)3]��4.0��10��38��Ksp[Cr(OH)3]��6.0��10��31)
��.(1)B��NaHAֻ�ܷ������룬���ܷ���ˮ��
(2)BC
(3)BD
��.(1)Cr2O72-��6Fe2����14H��===2Cr3����6Fe3����7H2O
(2)3.0��10��6mol��L��1
����

��һ����8�֣��ں������ʵ�����Ϊ0.1 mol FeCl3��H2SO4��Cu(NO3)2����Һ500 mL�У��������ۣ���Һ��n(Fe2��)�����n(Fe)�Ĺ�ϵ��ͼA(��ע��ʶͼ������ͼ��ÿС������Ϊ0.05mol)������ʾ����������ˮ�������Ӱ�죻��������������ԭʱ��������һ���������壩
��1��д��ͼ��n(Fe)��0.125mol-0.225 mol�ζ�Ӧ��Ӧ�����ӷ���ʽ____��
��2��д��ͼ��n(Fe)��0-0.05 mol�ζ�Ӧ��Ӧ�����ӷ���ʽ____��
��3������ͼB�л�����Һ��n(Fe3��)�����n(Fe)�ı仯��ϵͼ
��4������Ӧ���е����ȡ����Һ2mL�����Թ��У�Ϊ��֤������Һ����NO3�����ڣ��������Թ����ڵμ�_______��
| A������KMnO4��Һ | B�����������KSCN��Һ | C����ˮ | D������������Һ |
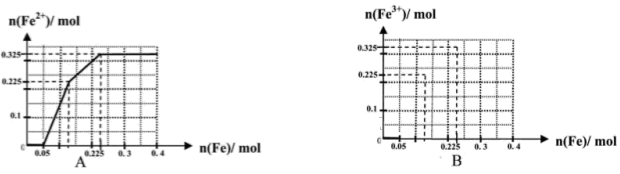
��������5�֣���ͼ�ǵ��۵�����ͼ����ͷ�ķ���ָ�����������Һ�����������ӽ���Ĥֻ����������ͨ������Ҫ�ô�װ����ͨ��������Na2SO4��NaOH�Ļ����Һ������װ����ͨ��A1��Һ��A2��Һ(A1��Һ��Na2SO4��NaOH�Ļ����Һ��A2��Һ��NaOH��ϡ��Һ)�����һ��ʱ���װ����������B2��Һ����NaOH��Ũ��Һ����B1��Һ��C1�����C2���塣
��1�� C2������______���ѧʽ����
��2����д��������Ӧ�ĵ缫����ʽ .
��3�������������������NaOH��Ũ��Һ��ԭ�� .
��.�ס���Ԫ�صĵ��ʺͻ�����Ӧ�ù㷺��
��1����Ԫ�ص�ԭ�ӽṹʾ��ͼ�� ��
��2��������뽹̿��ʯӢɰ���,�ڵ�¯�м��ȵ�1 500 �����ɰ���,��ӦΪ:
2Ca3��PO4��2+6SiO2 6CaSiO3+P4O10
6CaSiO3+P4O10
10C+P4O10 P4+10CO
P4+10CO
ÿ����1 mol P4ʱ,���� mol���ӷ���ת�ơ�
��3����������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6H8O6����ˮ��Һ�м������I2��Һ,ʹά����C��ȫ����,ʣ���I2��Na2S2O3��Һ�ζ�,�ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ:
C6H8O6+I2 C6H6O6+2H++2I-
C6H6O6+2H++2I-
2S2 +I2
+I2 S4
S4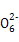 +2I-
+2I-
��һ�������ijά����C��Һ�м���a mol/L I2��ҺV1 mL,��ַ�Ӧ��,��Na2S2O3��Һ�ζ�ʣ���I2,����b mol/L Na2S2O3��ҺV2 mL������Һ��ά����C�����ʵ����� mol��
��4����������Һ��,����أ�KIO3�����������ƿɷ������·�Ӧ:
2I +5S
+5S +2H+
+2H+ I2+5S
I2+5S +H2O
+H2O
���ɵĵ�����õ�����Һ����,���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ:
| | 0.01 mol/LKIO3������Һ�������ۣ������/mL | 0.01 mol/LNa2SO3��Һ�����/mL | H2O�����/mL | ʵ���¶�/�� | ��Һ������ɫʱ����ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | |
| ʵ��2 | 5 | 5 | 40 | 25 | |
| ʵ��3 | 5 | 5 | V2 | 0 | |
��ʵ���Ŀ���� ;����V2= mL��
��.ϡ��Ԫ���DZ����ս����Դ,�ҹ����̲�����������λ��
��5���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�ء��ڼ���������CeCl3����ˮ��,��ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������,NH4Cl�������� ��
��6����ijǿ���Ի��ϡ����Һ�м���H2O2,����pH��3,Ce3+ͨ�����з�Ӧ�γ�Ce��OH��4�������Է��롣��ɷ�Ӧ�����ӷ���ʽ:
Ce3++H2O2+H2O
 Ce��OH��4��+ ��
Ce��OH��4��+ �� 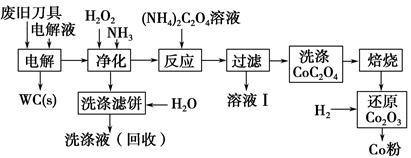
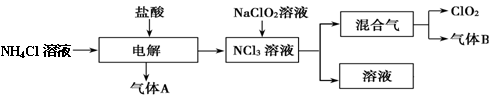
 Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
 4NH3 (g)+3O2 (g) - Q �������Ӧ��ƽ�ⳣ��Kֵ��÷�Ӧ ��ѡ���ţ���
4NH3 (g)+3O2 (g) - Q �������Ӧ��ƽ�ⳣ��Kֵ��÷�Ӧ ��ѡ���ţ���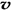 ��N2��/
��N2��/ 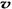 ��O2��=2:3
��O2��=2:3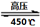 6H2O��+ 2N2��+ 4HCl��+ 5O2�����������������뻹ԭ��������ʵ���֮���� ��ÿ�ֽ�1mol������泥�ת�Ƶĵ�����Ŀ�� ��
6H2O��+ 2N2��+ 4HCl��+ 5O2�����������������뻹ԭ��������ʵ���֮���� ��ÿ�ֽ�1mol������泥�ת�Ƶĵ�����Ŀ�� ��