��Ŀ����
��10�֣���1�������ʽ��з���, ����ʶ���ʵ���ɡ��ṹ�����ʺ���;�ı��;�������÷���ķ�������ʶ�������ʣ�����ţ���
��NaCl���� �ڽ���ͭ ������ ��SO2 ������ �� BaSO4�ߴ�����
I �ܵ������____________��
II�����������ڵ���ʵ���____________��
III ���ڷǵ���ʵ���____________��
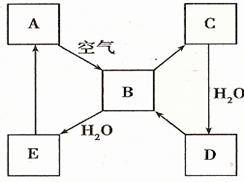
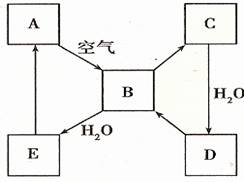
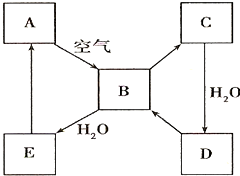
��2����ͼ��ʾij����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����B��C����Է����������16��������D����Ҫ�Ĺ�ҵԭ�ϡ�
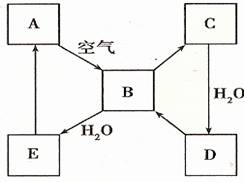
I ����A������ ��
II .д��D��Ũ��Һ��Cu���ȷ�Ӧ����B�Ļ�ѧ����ʽ________________��
(1). �ڢۣ�II __�٢ޢ�__��III. __�ܢ�__����ÿ��2�֣�
(2)I. �� ����2�֣�
II 2H2SO4��Cu CuSO4��2H2O��SO2����2�֣�
CuSO4��2H2O��SO2����2�֣�
����������1���������ʺͷǵ���ʵĸ������ˮ�������ۻ�ʱ�ܵ���Ļ��������ڵ���ʣ�����ˮ�������ۻ�ʱ�����ܵ���Ļ��������ڷǵ���ʡ�NaCl���塢BaSO4 �����������ڵ���ʣ��������磻����ͭ���Ե��磬���Ȳ����ڵ���ʣ�Ҳ�����ڷǵ���ʣ�������Ե��磬�����ڻ��� SO2 �����Dz����磬���߾����ڷǵ���ʡ�
��2���������仯��������ʡ���ѧ��ѧ�г����ĵ���ɫ�����ǹ������ƺ͵�������������A�ǵ�������ȼ�����ɶ�����������������ˮ����������H2SO3��H2SO3���ȶ����ֽ����ɶ��������ˮ��B��C����Է����������16������C����������������������ˮ�������ᣬD�����ᣬ���ᱻ��ԭ�������ɶ�������

 ��1�������ʽ��з��࣬����ʶ���ʵ���ɡ��ṹ�����ʺ���;�ı��;�������÷���ķ�������ʶ�������ʣ�����ţ�����NaCl���� �ڽ���ͭ ������ ��SO2 ������
��1�������ʽ��з��࣬����ʶ���ʵ���ɡ��ṹ�����ʺ���;�ı��;�������÷���ķ�������ʶ�������ʣ�����ţ�����NaCl���� �ڽ���ͭ ������ ��SO2 ������