��Ŀ����
(13��)��A��B��C��D���ֻ�����ֱ���K+��Ba2+��SO42����CO32����OH���е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ�
��1���ƶ�A��B��C��D��E�Ļ�ѧʽ��
A ��B ��C ��D ��E ��
��2��д�����з�Ӧ�����ӷ���ʽ��
B�����ᷴӦ
C�����ᷴӦ
D�����ᷴӦ
E�����ʯ��ˮ��Ӧ
(13��)��1��A��BaSO4 B��BaCO3 C: Ba(OH)2 D: K2CO3 E: CO2(ÿ��1��)
��2��2H����BaCO3��CO2����H2O��Ba2+
Ba2+ ��2H����2 OH����SO42����BaSO4����2H2O 2H����CO
2H����CO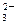 ��CO2����H2O
��CO2����H2O
Ca2+��2 OH����CO2��CaCO3����H2O(ÿ��2��)
����

������������⣺
(1)����ú�ͳɷ�֮һA�ķ����к���̼ԭ��n(A)���������ѻ�ΪB��C��������������зֱ�̼ԭ��n(B)��n(C)������BΪ�������������������ȷ����_____________________(����ţ���ѡ���ѡ���۷�)��
| n(A) n(B) n(C) |
a | 10 9 1 |
b | 11 6 5 |
c | 12 5 6 |
d | 13 9 3 |
e | 14 8 6 |
f | 15 11 4 |
(2)��д������b�����õ���ϩ���У���������4��̼ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��
(12��) ��A��B��C��D����ǿ����ʣ�������ˮ�е���ʱ�ɲ����������ӣ�(ÿ������ֻ��һ�������Ӻ�һ���������һ����ظ�)
|
������ |
Na+��Ba2+��NH4+��K+ |
|
������ |
CH3COO����Cl����OH����SO42�� |
��֪����A��C��Һ��pH������7��B��Һ��pHС��7��A��B��Һ��ˮ�ĵ���̶���ͬ��D��Һ��ɫ��Ӧ�Ի�ɫ��
��C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ�����д̼�����ζ�����壬A��Һ��D��Һ���ʱ����������
(1)A��������____________��
(2)�����ӷ���ʽ��ʾA��ˮ��Һ�Լ��Ե�ԭ��______________________________��
(3)25 ��ʱpH��9��A��Һ��pH��9��C��Һ��ˮ�ĵ���̶Ƚ�С����________(��дA��C�Ļ�ѧʽ)��
(4)25 ��ʱ�ö��Ե缫���D��ˮ��Һ��һ��ʱ�����Һ��pH________7(�>������<������)��
(5)��������������ʵ���Ũ�ȵ�B��Һ��C��Һ��ϣ���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊ__________________________________________________��
(6)����ʱ��һ�����0.2 mol��L��1��C��Һ�У�����һ�������0.1 mol��L��1������ʱ�������Һ��pH��13������Ӧ����Һ���������C��Һ����������֮�ͣ���C��Һ��������������________��